Comparing Salivary Caffeine Kinetics of 13C and 12C Caffeine for Gastric Emptying of 50 mL Water
- PMID: 36839650
- PMCID: PMC9963808
- DOI: 10.3390/pharmaceutics15020328
Comparing Salivary Caffeine Kinetics of 13C and 12C Caffeine for Gastric Emptying of 50 mL Water
Abstract
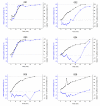
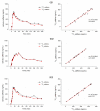
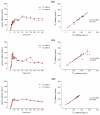
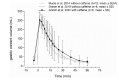
Gastric water emptying as a critical parameter for oral drug absorption can be investigated by several imaging techniques or by the interpretation of pharmacokinetics of appropriate substances. Recently introduced salivary caffeine kinetics is a valuable tool, but the required caffeine abstinence limits its applicability. To avoid the caffeine abstinence, stable isotope-labeled caffeine might be used, but the representability and transferability of kinetics for evaluation of gastric emptying must be demonstrated. Thus, salivary caffeine pharmacokinetics were compared for naturally occurring 12C-caffeine and 13C3-caffeine after the administration of water under fasting conditions in six healthy young subjects. For this purpose, an ice capsule containing the two caffeine species was administered with 50 mL tap water. Gastric water emptying was simultaneously quantified using magnetic resonance imaging (MRI). Gastric emptying of 50 mL of water could be successfully evaluated. The salivary caffeine kinetics of 13C3- and 12C-caffeine were nearly congruent and showed good linear correlations in all subjects, with a mean correlation coefficient of 0.96 in pooled data. Thus, the substitution of natural 12C caffeine with stable isotope-labeled 13C3-caffeine offers the opportunity for broader application of the salivary caffeine gastric emptying technique and increases the robustness of the method against environmental contamination with caffeine.
Keywords: UNGAP; caffeine; gastric emptying; magnetic resonance imaging (MRI); saliva tracers; stable isotopes.
Conflict of interest statement
M.G., A.R., L.M., R.K., E.S., M.T. and W.W. are only affiliated with the University of Greifswald and declare no conflicts of interest. M.F. is now an employee of Bayer AG, but the company was not involved in the study design, conduction or evaluation. M.F. also declares no conflicts of interest.
Figures

Similar articles
-
Determination of Gastric Water Emptying in Fasted and Fed State Conditions Using a Compression-Coated Tablet and Salivary Caffeine Kinetics.Pharmaceutics. 2023 Nov 4;15(11):2584. doi: 10.3390/pharmaceutics15112584. Pharmaceutics. 2023. PMID: 38004563 Free PMC article.
-
Low dose caffeine as a salivary tracer for the determination of gastric water emptying in fed and fasted state: A MRI validation study.Eur J Pharm Biopharm. 2018 Jun;127:443-452. doi: 10.1016/j.ejpb.2018.03.011. Epub 2018 Mar 27. Eur J Pharm Biopharm. 2018. PMID: 29602018
-
Comparing the gastric emptying of 240 mL and 20 mL water by MRI and caffeine salivary tracer technique.Eur J Pharm Biopharm. 2023 Mar;184:150-158. doi: 10.1016/j.ejpb.2023.01.021. Epub 2023 Feb 1. Eur J Pharm Biopharm. 2023. PMID: 36736963
-
Combined Application of MRI and the Salivary Tracer Technique to Determine the in Vivo Disintegration Time of Immediate Release Formulation Administered to Healthy, Fasted Subjects.Mol Pharm. 2019 Apr 1;16(4):1782-1786. doi: 10.1021/acs.molpharmaceut.8b01320. Epub 2019 Mar 8. Mol Pharm. 2019. PMID: 30821987
-
Impact of advanced age on the gastric emptying of water under fasted and fed state conditions.Eur J Pharm Sci. 2024 Oct 1;201:106853. doi: 10.1016/j.ejps.2024.106853. Epub 2024 Jul 20. Eur J Pharm Sci. 2024. PMID: 39033883
Cited by
-
Quantitative analysis of gastrointestinal fluid absorption and secretion to estimate luminal fluid dynamics in rats.Sci Rep. 2023 Oct 14;13(1):17454. doi: 10.1038/s41598-023-44742-y. Sci Rep. 2023. PMID: 37838772 Free PMC article.
-
Does the appearance of the Magenstrasse depend on the amount of water consumed?Int J Pharm X. 2025 Jul 23;10:100365. doi: 10.1016/j.ijpx.2025.100365. eCollection 2025 Dec. Int J Pharm X. 2025. PMID: 40761844 Free PMC article.
-
Determination of Gastric Water Emptying in Fasted and Fed State Conditions Using a Compression-Coated Tablet and Salivary Caffeine Kinetics.Pharmaceutics. 2023 Nov 4;15(11):2584. doi: 10.3390/pharmaceutics15112584. Pharmaceutics. 2023. PMID: 38004563 Free PMC article.
References
-
- Hens B., Corsetti M., Spiller R., Marciani L., Vanuytsel T., Tack J., Talattof A., Amidon G., Koziolek M., Weitschies W., et al. Exploring Gastrointestinal Variables Affecting Drug and Formulation Behavior: Methodologies, Challenges and Opportunities. Int. J. Pharm. 2016;519:79–97. doi: 10.1016/j.ijpharm.2016.11.063. - DOI - PubMed
-
- Grimm M., Aude P., Feldmüller M., Kessler R., Scheuch E., Tzvetkov M., Koziolek M., Weitschies W. Comparing the gastric emptying of 240 mL and 20 mL water by MRI and caffeine salivary tracer technique. Eur. J. Pharm. Biopharm. 2023 under review. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

