Biomaterials Based on Organic Polymers and Layered Double Hydroxides Nanocomposites: Drug Delivery and Tissue Engineering
- PMID: 36839735
- PMCID: PMC9961265
- DOI: 10.3390/pharmaceutics15020413
Biomaterials Based on Organic Polymers and Layered Double Hydroxides Nanocomposites: Drug Delivery and Tissue Engineering
Abstract
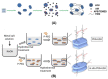
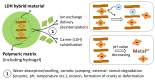
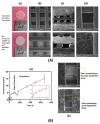
The development of biomaterials has a substantial role in pharmaceutical and medical strategies for the enhancement of life quality. This review work focused on versatile biomaterials based on nanocomposites comprising organic polymers and a class of layered inorganic nanoparticles, aiming for drug delivery (oral, transdermal, and ocular delivery) and tissue engineering (skin and bone therapies). Layered double hydroxides (LDHs) are 2D nanomaterials that can intercalate anionic bioactive species between the layers. The layers can hold metal cations that confer intrinsic biological activity to LDHs as well as biocompatibility. The intercalation of bioactive species between the layers allows the formation of drug delivery systems with elevated loading capacity and modified release profiles promoted by ion exchange and/or solubilization. The capacity of tissue integration, antigenicity, and stimulation of collagen formation, among other beneficial characteristics of LDH, have been observed by in vivo assays. The association between the properties of biocompatible polymers and LDH-drug nanohybrids produces multifunctional nanocomposites compatible with living matter. Such nanocomposites are stimuli-responsive, show appropriate mechanical properties, and can be prepared by creative methods that allow a fine-tuning of drug release. They are processed in the end form of films, beads, gels, monoliths etc., to reach orientated therapeutic applications. Several studies attest to the higher performance of polymer/LDH-drug nanocomposite compared to the LDH-drug hybrid or the free drug.
Keywords: anionic clays; composite biomaterials; drug delivery system; hydrotalcite; intercalation compounds; layered double hydroxides; layered materials; nano-based drug carrier; nanocomposites; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

















References
-
- [(accessed on 18 November 2022)]. Available online: https://sustainabledevelopment.un.org/outcomedocuments/agenda21.
-
- Ghasemi-Mobarakeh L., Kolahreez D., Ramakrishna S., Williams D. Key Terminology in Biomaterials and Biocompatibility. Curr. Opin. Biomed. Eng. 2019;10:45–50. doi: 10.1016/j.cobme.2019.02.004. - DOI
-
- IUPAC . In: Compendium of Chemical Terminology. 2nd ed. McNaught A.D., Wilkinson A., editors. Blackwell Scientific Publications; Oxford, UK: 1997. the “Gold Book”.
Publication types
Grants and funding
- 88887.352040/2019-00/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
- 314034/2021-8/National Council for Scientific and Technological Development
- 425730/2018-2; 315109/2021-1; 401840/2021-2; 405048/2021-1/National Council for Scientific and Technological Development
- 33002010191P0/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
- 2014/50869-6/São Paulo Research Foundation
LinkOut - more resources
Full Text Sources

