A Theranostic Small-Molecule Prodrug Conjugate for Neuroendocrine Prostate Cancer
- PMID: 36839802
- PMCID: PMC9967013
- DOI: 10.3390/pharmaceutics15020481
A Theranostic Small-Molecule Prodrug Conjugate for Neuroendocrine Prostate Cancer
Abstract
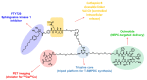
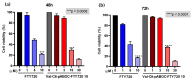
After androgen deprivation therapy, a significant number of prostate cancer cases progress with a therapy-resistant neuroendocrine phenotype (NEPC). This represents a challenge for diagnosis and treatment. Based on our previously reported design of theranostic small-molecule prodrug conjugates (T-SMPDCs), herein we report a T-SMPDC tailored for targeted positron emission tomography (PET) imaging and chemotherapy of NEPC. The T-SMPDC is built upon a triazine core (TZ) to present three functionalities: (1) a chelating moiety (DOTA: 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) for PET imaging when labeled with 68Ga (t1/2 = 68 min) or other relevant radiometals; (2) an octreotide (Octr) that targets the somatostatin receptor 2 (SSTR2), which is overexpressed in the innervated tumor microenvironment (TME); and (3) fingolimod, FTY720-an antagonist of sphingosine kinase 1 that is an intracellular enzyme upregulated in NEPC. Polyethylene glycol (PEG) chains were incorporated via conventional conjugation methods or a click chemistry reaction forming a 1,4-disubstituted 1,2,3-triazole (Trz) linkage for the optimization of in vivo kinetics as necessary. The T-SMPDC, DOTA-PEG3-TZ(PEG4-Octr)-PEG2-Trz-PEG3-Val-Cit-pABOC-FTY720 (PEGn: PEG with n repeating ethyleneoxy units (n = 2, 3, or 4); Val: valine; Cit: citrulline; pABOC: p-amino-benzyloxycarbonyl), showed selective SSTR2 binding and mediated internalization of the molecule in SSTR2 high cells. Release of FTY720 was observed when the T-SMPDC was exposed to cathepsin B, and the released FTY720 exerted cytotoxicity in cells. In vivo PET imaging showed significantly higher accumulation (2.1 ± 0.3 %ID/g; p = 0.02) of [68Ga]Ga-DOTA-PEG3-TZ(PEG4-Octr)-PEG2-Trz-PEG3-Val-Cit-pABOC-FTY720 in SSTR2high prostate cancer xenografts than in the SSTR2low xenografts (1.5 ± 0.4 %ID/g) at 13 min post-injection (p.i.) with a rapid excretion through the kidneys. Taken together, these proof-of-concept results validate the design concept of the T-SMPDC, which may hold a great potential for targeted diagnosis and therapy of NEPC.
Keywords: controlled drug release; drug delivery; neuroendocrine prostate cancer; positron emission tomography (PET); prodrug conjugate; targeted therapy; theranostics; tumor innervation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Theranostic Small-Molecule Prodrug Conjugates for Targeted Delivery and Controlled Release of Toll-like Receptor 7 Agonists.Int J Mol Sci. 2022 Jun 28;23(13):7160. doi: 10.3390/ijms23137160. Int J Mol Sci. 2022. PMID: 35806163 Free PMC article.
-
Design of a Small-Molecule Drug Conjugate for Prostate Cancer Targeted Theranostics.Bioconjug Chem. 2016 Jul 20;27(7):1681-9. doi: 10.1021/acs.bioconjchem.6b00222. Epub 2016 Jun 15. Bioconjug Chem. 2016. PMID: 27248781
-
More advantages in detecting bone and soft tissue metastases from prostate cancer using 18F-PSMA PET/CT.Hell J Nucl Med. 2019 Jan-Apr;22(1):6-9. doi: 10.1967/s002449910952. Epub 2019 Mar 7. Hell J Nucl Med. 2019. PMID: 30843003
-
The Search for an Alternative to [68Ga]Ga-DOTA-TATE in Neuroendocrine Tumor Theranostics: Current State of 18F-labeled Somatostatin Analog Development.Theranostics. 2019 Feb 14;9(5):1336-1347. doi: 10.7150/thno.31806. eCollection 2019. Theranostics. 2019. PMID: 30867834 Free PMC article. Review.
-
Radiopharmaceuticals for the Diagnosis and Therapy of Neuroendocrine Differentiated Prostate Cancer.Curr Radiopharm. 2017;10(1):6-15. doi: 10.2174/1874471009666161229123126. Curr Radiopharm. 2017. PMID: 28034291 Review.
Cited by
-
Targeting Sphingosine 1-Phosphate Metabolism as a Therapeutic Avenue for Prostate Cancer.Cancers (Basel). 2023 May 12;15(10):2732. doi: 10.3390/cancers15102732. Cancers (Basel). 2023. PMID: 37345069 Free PMC article. Review.
-
Decreased MARCKS Protein Expression in Kidney Cortex Membrane Fractions of Cathepsin B Knockout Mice Is Associated with Reduced Lysophosphatidylcholine and Protein Kinase C Activity.Biomedicines. 2023 May 20;11(5):1489. doi: 10.3390/biomedicines11051489. Biomedicines. 2023. PMID: 37239160 Free PMC article.
-
Aggressive PitNETs and Potential Target Therapies: A Systematic Review of Molecular and Genetic Pathways.Int J Mol Sci. 2023 Oct 29;24(21):15719. doi: 10.3390/ijms242115719. Int J Mol Sci. 2023. PMID: 37958702 Free PMC article.
-
Nanomicellar Prodrug Delivery of Glucose-Paclitaxel: A Strategy to Mitigate Paclitaxel Toxicity.Int J Nanomedicine. 2025 Feb 17;20:2087-2101. doi: 10.2147/IJN.S500999. eCollection 2025. Int J Nanomedicine. 2025. PMID: 39990288 Free PMC article.
-
Targeting Key Players of Neuroendocrine Differentiation in Prostate Cancer.Int J Mol Sci. 2023 Sep 5;24(18):13673. doi: 10.3390/ijms241813673. Int J Mol Sci. 2023. PMID: 37761978 Free PMC article. Review.
References
-
- Tosoian J.J., Gorin M.A., Rowe S.P., Andreas D., Szabo Z., Pienta K.J., Pomper M.G., Lotan T.L., Ross A.E. Correlation of PSMA-Targeted 18F-DCFPyL PET/CT Findings with Immunohistochemical and Genomic Data in a Patient with Metastatic Neuroendocrine Prostate Cancer. Clin. Genitourin. Cancer. 2017;15:e65–e68. doi: 10.1016/j.clgc.2016.09.002. - DOI - PMC - PubMed
-
- Parida G.K., Tripathy S., Datta Gupta S., Singhal A., Kumar R., Bal C., Shamim S.A. Adenocarcinoma Prostate with Neuroendocrine Differentiation: Potential Utility of 18F-FDG PET/CT and 68Ga-DOTANOC PET/CT Over 68Ga-PSMA PET/CT. Clin. Nucl. Med. 2018;43:248–249. doi: 10.1097/RLU.0000000000002013. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

