Cancer Treatment Using Different Shapes of Gold-Based Nanomaterials in Combination with Conventional Physical Techniques
- PMID: 36839822
- PMCID: PMC9968101
- DOI: 10.3390/pharmaceutics15020500
Cancer Treatment Using Different Shapes of Gold-Based Nanomaterials in Combination with Conventional Physical Techniques
Abstract
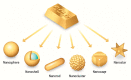
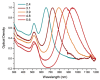
The conventional methods of cancer treatment and diagnosis, such as radiotherapy, chemotherapy, and computed tomography, have developed a great deal. However, the effectiveness of such methods is limited to the possible failure or collateral effects on the patients. In recent years, nanoscale materials have been studied in the field of medical physics to develop increasingly efficient methods to treat diseases. Gold nanoparticles (AuNPs), thanks to their unique physicochemical and optical properties, were introduced to medicine to promote highly effective treatments. Several studies have confirmed the advantages of AuNPs such as their biocompatibility and the possibility to tune their shapes and sizes or modify their surfaces using different chemical compounds. In this review, the main properties of AuNPs are analyzed, with particular focus on star-shaped AuNPs. In addition, the main methods of tumor treatment and diagnosis involving AuNPs are reviewed.
Keywords: cancer; diagnostics; gold nanoparticles; medical physics; nanomedicine; nanostars; therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













References
-
- Nejati K., Dadashpour M., Gharibi T., Mellatyar H., Akbarzadeh A. Biomedical applications of functionalized gold nanoparticles: A review. J. Clust. Sci. 2022;33:1–16. doi: 10.1007/s10876-020-01955-9. - DOI
-
- Huang X., El-Sayed M.A. Plasmonic photo-thermal therapy (PPTT) Alex. J. Med. 2011;47:1–9. doi: 10.1016/j.ajme.2011.01.001. - DOI
Publication types
LinkOut - more resources
Full Text Sources

