Cannabidiol-Loaded Extracellular Vesicles from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Paclitaxel-Induced Peripheral Neuropathy
- PMID: 36839877
- PMCID: PMC9964872
- DOI: 10.3390/pharmaceutics15020554
Cannabidiol-Loaded Extracellular Vesicles from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Paclitaxel-Induced Peripheral Neuropathy
Erratum in
-
Correction: Kalvala et al. Cannabidiol-Loaded Extracellular Vesicles from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Paclitaxel-Induced Peripheral Neuropathy. Pharmaceutics 2023, 15, 554.Pharmaceutics. 2023 Aug 25;15(9):2200. doi: 10.3390/pharmaceutics15092200. Pharmaceutics. 2023. PMID: 37765336 Free PMC article.
Abstract
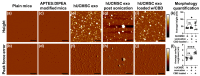
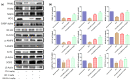
In cancer patients, chronic paclitaxel (PTX) treatment causes excruciating pain, limiting its use in cancer chemotherapy. The neuroprotective potential of synthetic cannabidiol (CBD) and CBD formulated in extracellular vesicles (CBD-EVs) isolated from human umbilical cord derived mesenchymal stem cells was investigated in C57BL/6J mice with PTX-induced neuropathic pain (PIPN). The particle size of EVs and CBD-EVs, surface roughness, nanomechanical properties, stability, and release studies were all investigated. To develop neuropathy in mice, PTX (8 mg/kg, i.p.) was administered every other day (four doses). In terms of decreasing mechanical and thermal hypersensitivity, CBD-EVs treatment was superior to EVs treatment or CBD treatment alone (p < 0.001). CBD and CBD-EVs significantly reduced mitochondrial dysfunction in dorsal root ganglions and spinal homogenates of PTX-treated animals by modulating the AMPK pathway (p < 0.001). Studies inhibiting the AMPK and 5HT1A receptors found that CBD did not influence the neurobehavioral or mitochondrial function of PIPN. Based on these results, we hypothesize that CBD and CBD-EVs mitigated PIPN by modulating AMPK and mitochondrial function.
Keywords: AMPK; CBD-EVs; Young’s modulus; atomic force microscopy; extracellular vesicles; hUCMSCs; morphology.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures






References
-
- Rugo H.S., Barry W.T., Moreno-Aspitia A., Lyss A.P., Cirrincione C., Leung E., Mayer E.L., Naughton M., Toppmeyer D., Carey L.A., et al. Randomized phase III trial of paclitaxel once per week compared with nanoparticle albumin-bound nab-paclitaxel once per week or ixabepilone with bevacizumab as first-line chemotherapy for locally recurrent or metastatic breast cancer: CALGB 40502/NCCTG N063H (Alliance) J. Clin. Oncol. 2015;33:2361. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

