Cyclosporine A-Loaded Ternary Solid Dispersion Prepared with Fine Droplet Drying Process for Improvement of Storage Stability and Oral Bioavailability
- PMID: 36839893
- PMCID: PMC9965122
- DOI: 10.3390/pharmaceutics15020571
Cyclosporine A-Loaded Ternary Solid Dispersion Prepared with Fine Droplet Drying Process for Improvement of Storage Stability and Oral Bioavailability
Abstract
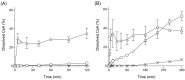
This study aimed to develop a cyclosporine A (CsA)-loaded ternary solid dispersion (tSD/CsA) to improve the storage stability of a solid dispersion (SD) system and the oral absorbability of CsA. Hydroxypropyl cellulose (HPC) and hydroxypropyl methylcellulose acetate succinate (HPMCAS) were selected as carrier materials of tSD, and tSD/CsA was prepared with a fine droplet drying process, a powderization technology that employs an inkjet head. The physicochemical properties of tSD/CsA were evaluated in terms of morphology, storage stability, dissolution behavior, and mucoadhesive property. After the oral administration of CsA samples (10 mg-CsA/kg) to rats, the plasma concentration of CsA was monitored to estimate oral absorbability. tSD/CsA comprised uniform shriveled particles with a diameter of 3.4 mm and span factor of 0.4, which is a parameter to estimate the particle size distribution. Although HPC-based binary SD showed marked aggregation of the particles after storage under 40 °C/75% relative humidity, there were no significant aggregations of tSD/CsA, due to the relatively low hygroscopic property of HPMCAS. The pH-dependent release of CsA with improved dissolution was observed in tSD/CsA. In the in vitro mucoadhesive evaluation using a mucin disk, tSD/CsA exhibited a better mucoadhesive property than HPC-based SD, possibly leading to prolonged retention of tSD particles in the gastrointestinal tract after oral administration. Orally-dosed tSD/CsA in rats resulted in significantly improved oral absorption of CsA, as evidenced by a 27-fold higher bioavailability than amorphous CsA. tSD/CsA may be a promising dosage option to improve the storage stability of a SD system and the biopharmaceutical properties of CsA.
Keywords: cyclosporine A; fine droplet drying process; mucoadhesive particle; oral absorption; storage stability; ternary solid dispersion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Amorphous solid dispersion of cyclosporine A prepared with fine droplet drying process: Physicochemical and pharmacokinetic characterization.Int J Pharm. 2017 Mar 15;519(1-2):213-219. doi: 10.1016/j.ijpharm.2017.01.018. Epub 2017 Jan 16. Int J Pharm. 2017. PMID: 28093327
-
Self-micellizing solid dispersion of cyclosporine A with improved dissolution and oral bioavailability.Eur J Pharm Sci. 2014 Oct 1;62:16-22. doi: 10.1016/j.ejps.2014.05.006. Epub 2014 May 14. Eur J Pharm Sci. 2014. PMID: 24836392
-
Comparative studies on physicochemical stability of cyclosporine A-loaded amorphous solid dispersions.Int J Pharm. 2012 Apr 15;426(1-2):302-306. doi: 10.1016/j.ijpharm.2012.01.022. Epub 2012 Jan 20. Int J Pharm. 2012. PMID: 22285473
-
Hydroxypropyl methylcellulose acetate succinate as an exceptional polymer for amorphous solid dispersion formulations: A review from bench to clinic.Eur J Pharm Biopharm. 2022 Aug;177:289-307. doi: 10.1016/j.ejpb.2022.07.010. Epub 2022 Jul 21. Eur J Pharm Biopharm. 2022. PMID: 35872180 Review.
-
[Strategic formulation study on dry powder inhalation system based on modulated molecular properties and controlled pharmacokinetics].Yakugaku Zasshi. 2013;133(1):93-8. doi: 10.1248/yakushi.12-00209. Yakugaku Zasshi. 2013. PMID: 23292025 Review. Japanese.
References
-
- Sato H., Tabata A., Moritani T., Morinaga T., Mizumoto T., Seto Y., Onoue S. Design and Characterizations of Inhalable Poly(lactic-co-glycolic acid) Microspheres Prepared by the Fine Droplet Drying Process for a Sustained Effect of Salmon Calcitonin. Molecules. 2020;25:1311. doi: 10.3390/molecules25061311. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

