Ultrasonically Fabricated Beta-Carotene Nanoemulsion: Optimization, Characterization and Evaluation of Combinatorial Effect with Quercetin on Streptozotocin-Induced Diabetic Rat Model
- PMID: 36839896
- PMCID: PMC9962907
- DOI: 10.3390/pharmaceutics15020574
Ultrasonically Fabricated Beta-Carotene Nanoemulsion: Optimization, Characterization and Evaluation of Combinatorial Effect with Quercetin on Streptozotocin-Induced Diabetic Rat Model
Abstract
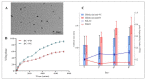
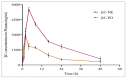
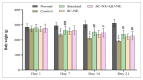
Diabetes mellitus (D.M.) is a metabolic disease that has affected over 500 million people globally. Bioactive compounds such as β-carotene and Quercetin have gained research interest for their potential antidiabetic properties, and bioactives have reported superior combinatorial effects in several ailments, including D.M. However, poor oral bioavailability has limited their potential application. Thus, the present study was focused on developing ultrasonically fabricated β-Carotene nanoemulsion (βC-NE) by employing capmul as the oil phase, Gelucire 44/14 as surfactant and Acconon MCM C8 as co-surfactant. The 3 factor- 3 level Box-Behnken design (BBD) was applied to optimise the βC-NE and study the impact of selected independent variables such as % Smix (5 to 9%), amplitude (20-30%) and sonication time (2.5-7.5 min) on responses including globule size (G.S.), poly dispersibility Index (PDI) and entrapment efficiency (E.E.). Further, the combinatorial effect of βC-NE with Quercetin Nanoemulsion (QU-NE) in the streptozotocin-induced diabetic rat model was evaluated. The results exhibited that 7% Smix at 25% amplitude for 5 min produced βC-NE with a droplet size of 153.1 ± 12.25 nm, 0.200 ± 0.04 PDI, and 73.25 ± 3.25% E.E. The βC-NE showed superior in-vivo bioavailability by 5.38 folds. The βC-NE, combined with QU-NE, exhibited potential therapeutic benefits in controlling body weight, blood sugar level, lipid levels, and tissue damage markers. Additionally, the pancreatic cells and hepatic cells were well protected. These results demonstrate the potential benefits of βC-NE and QU-NE in combination and recommend them as a substitute strategy for diabetes.
Keywords: Box-Behnken design; beta-carotene; diabetes mellitus; nanoemulsion; quercetin; ultrasonication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Asemi Z., Alizadeh S.A., Ahmad K., Goli M., Esmaillzadeh A. Effects of beta-carotene fortified synbiotic food on metabolic control of patients with type 2 diabetes mellitus: A double-blind randomized cross-over controlled clinical trial. Clin. Nutr. 2016;35:819–825. doi: 10.1016/j.clnu.2015.07.009. - DOI - PubMed
LinkOut - more resources
Full Text Sources

