Blockage of the IL-31 Pathway as a Potential Target Therapy for Atopic Dermatitis
- PMID: 36839897
- PMCID: PMC9961325
- DOI: 10.3390/pharmaceutics15020577
Blockage of the IL-31 Pathway as a Potential Target Therapy for Atopic Dermatitis
Abstract
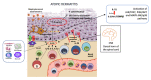
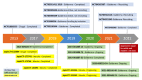
Atopic dermatitis (AD), a pruritic, inflammatory chronic disease with multifactorial pathogenesis, has been a therapeutic challenge. Novel target treatments aim to reduce not only the immunologic dysfunction and microbiome dysbiosis but also the recovery of the damaged skin barrier. The current review focuses on the interleukin 31 (IL-31) pathway and AD and offers an overview of the current clinical studies with monoclonal antibodies blocking this cascade. Pruritus, the key symptom of AD, has substantial participation of the IL-31 complex and activation of relevant signaling pathways. Epidermal keratinocytes, inflammatory cells, and cutaneous peripheral nerves express the interleukin-31 receptor α-chain (IL-31RA), upregulated by Staphylococcus aureus toxins or Th2 cytokines involved in AD. Nemolizumab is a humanized monoclonal antibody that antagonizes IL-31RA, inhibiting the IL-31 cascade and therefore contributing to reducing the pruritus and inflammation and recovering the damaged skin barrier in AD patients. Phases 2 and 3 clinical trials with nemolizumab in AD show a suitable safety profile, with a fast, efficient, and sustained reduction of pruritus and severity scores, especially when associated with topical treatment. Deciphering the full interplay of the IL-31 pathway and AD may expand the potential of nemolizumab as a targeted therapy for AD and other pruritic conditions.
Keywords: atopic dermatitis; interleukin-31 (IL-31); interleukin-31 receptor α-chain (IL-31RA); pruritus.
Conflict of interest statement
R.L.O. has been an investigator and/or consultant for Bayer, Eli Lilly; V.A. has been an investigator and/or consultant to Abbvie, Eli Lilly, Pfizer. These had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Zeze N., Kido-Nakahara M., Tsuji G., Maehara E., Sato Y., Sakai S., Fujishima K., Hashimoto-Hachiya A., Furue M., Nakahara T. Role of ERK Pathway in the Pathogenesis of Atopic Dermatitis and Its Potential as a Therapeutic Target. Int. J. Mol. Sci. 2022;23:3467. doi: 10.3390/ijms23073467. - DOI - PMC - PubMed
-
- Brunner P.M., Israel A., Zhang N., Leonard A., Wen H.C., Huynh T., Tran G., Lyon S., Rodriguez G., Immaneni S., et al. Early-onset pediatric atopic dermatitis is characterized by T(H)2/T(H)17/T(H)22-centered inflammation and lipid alterations. J. Allergy Clin. Immunol. 2018;141:2094–2106. doi: 10.1016/j.jaci.2018.02.040. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

