A Systematic Overview of Eudragit® Based Copolymer for Smart Healthcare
- PMID: 36839910
- PMCID: PMC9962897
- DOI: 10.3390/pharmaceutics15020587
A Systematic Overview of Eudragit® Based Copolymer for Smart Healthcare
Abstract
Eudragit, synthesized by radical polymerization, is used for enteric coating, precise temporal release, and targeting the entire gastrointestinal system. Evonik Healthcare Germany offers different grades of Eudragit. The ratio of methacrylic acid to its methacrylate-based monomers used in the polymerization reaction defines the final product's characteristics and consequently its potential range of applications. Since 1953, these polymers have been made to use in a wide range of healthcare applications around the world. In this review, we reviewed the "known of knowns and known of unknowns" about Eudragit, from molecule to material design, its characterization, and its applications in healthcare.
Keywords: Eudragit classification; Eudragit synthesis; biosensor; drug delivery; nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





















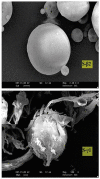
References
-
- Shah H., Jain A., Laghate G., Prabhudesai D. Remington: The Science and Practice of Pharmacy. Academic Press; Cambridge, MA, USA: 2020. Pharmaceutical Excipients; pp. 633–643. - DOI
-
- Patra C.N., Priya R., Swain S., Kumar Jena G., Panigrahi K.C., Ghose D. Pharmaceutical Significance of Eudragit: A Review. Futur. J. Pharm. Sci. 2017;3:33–45. doi: 10.1016/j.fjps.2017.02.001. - DOI
-
- Wen H., Park K. Oral Controlled Release Formulation Design and Drug Delivery: Theory to Practice. John Wiley & Sons; Hoboken, NJ, USA: 2010. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

