Commercially Available Cell-Free Permeability Tests for Industrial Drug Development: Increased Sustainability through Reduction of In Vivo Studies
- PMID: 36839914
- PMCID: PMC9964961
- DOI: 10.3390/pharmaceutics15020592
Commercially Available Cell-Free Permeability Tests for Industrial Drug Development: Increased Sustainability through Reduction of In Vivo Studies
Abstract
Replacing in vivo with in vitro studies can increase sustainability in the development of medicines. This principle has already been applied in the biowaiver approach based on the biopharmaceutical classification system, BCS. A biowaiver is a regulatory process in which a drug is approved based on evidence of in vitro equivalence, i.e., a dissolution test, rather than on in vivo bioequivalence. Currently biowaivers can only be granted for highly water-soluble drugs, i.e., BCS class I/III drugs. When evaluating poorly soluble drugs, i.e., BCS class II/IV drugs, in vitro dissolution testing has proved to be inadequate for predicting in vivo drug performance due to the lack of permeability interpretation. The aim of this review was to provide solid proofs that at least two commercially available cell-free in vitro assays, namely, the parallel artificial membrane permeability assay, PAMPA, and the PermeaPad® assay, PermeaPad, in different formats and set-ups, have the potential to reduce and replace in vivo testing to some extent, thus increasing sustainability in drug development. Based on the literature review presented here, we suggest that these assays should be implemented as alternatives to (1) more energy-intense in vitro methods, e.g., refining/replacing cell-based permeability assays, and (2) in vivo studies, e.g., reducing the number of pharmacokinetic studies conducted on animals and humans. For this to happen, a new and modern legislative framework for drug approval is required.
Keywords: PAMPA; PermeaPad®; biowaiver; dissolution-permeation; in vitro AUC; permeability; unstirred water layer.
Conflict of interest statement
Massimiliano Pio di Cagno, the corresponding and leading author of this review, is Scientific Consultant for InnoME-Phabioc GmbH, the producer of the PermeaPad. Ann-Christin Jacobsen’s doctoral studies were partly financed by InnoME GmbH. In order to minimize the risk of having a biased scientific narrative, the list of authors included experts who have been working/currently are working with PAMPA systems (Sonja Visentin and Cosmin Butnarasu).
Figures




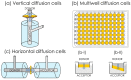
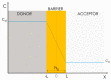



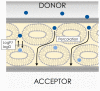





References
-
- Belkhir L., Elmeligi A. Carbon footprint of the global pharmaceutical industry and relative impact of its major players. J. Clean. Prod. 2019;214:185–194. doi: 10.1016/j.jclepro.2018.11.204. - DOI
-
- Forin S., Scholz R. Are medicines more greenhouse gas intensive than cars? Comment to Belkhir, L., Elmeligi, A., 2019: Carbon footprint of the global pharmaceutical industry and relative impact of its major players. J. Clean. Prod. 2022;331:129963. doi: 10.1016/j.jclepro.2021.129963. - DOI
-
- Milanesi M., Runfola A., Guercini S. Pharmaceutical industry riding the wave of sustainability: Review and opportunities for future research. J. Clean. Prod. 2020;261:121204. doi: 10.1016/j.jclepro.2020.121204. - DOI
Publication types
LinkOut - more resources
Full Text Sources

