A Review of Protein- and Peptide-Based Chemical Conjugates: Past, Present, and Future
- PMID: 36839922
- PMCID: PMC9959917
- DOI: 10.3390/pharmaceutics15020600
A Review of Protein- and Peptide-Based Chemical Conjugates: Past, Present, and Future
Abstract
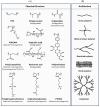
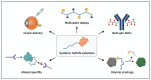
Over the past few decades, the complexity of molecular entities being advanced for therapeutic purposes has continued to evolve. A main propellent fueling innovation is the perpetual mandate within the pharmaceutical industry to meet the needs of novel disease areas and/or delivery challenges. As new mechanisms of action are uncovered, and as our understanding of existing mechanisms grows, the properties that are required and/or leveraged to enable therapeutic development continue to expand. One rapidly evolving area of interest is that of chemically enhanced peptide and protein therapeutics. While a variety of conjugate molecules such as antibody-drug conjugates, peptide/protein-PEG conjugates, and protein conjugate vaccines are already well established, others, such as antibody-oligonucleotide conjugates and peptide/protein conjugates using non-PEG polymers, are newer to clinical development. This review will evaluate the current development landscape of protein-based chemical conjugates with special attention to considerations such as modulation of pharmacokinetics, safety/tolerability, and entry into difficult to access targets, as well as bioavailability. Furthermore, for the purpose of this review, the types of molecules discussed are divided into two categories: (1) therapeutics that are enhanced by protein or peptide bioconjugation, and (2) protein and peptide therapeutics that require chemical modifications. Overall, the breadth of novel peptide- or protein-based therapeutics moving through the pipeline each year supports a path forward for the pursuit of even more complex therapeutic strategies.
Keywords: ADC; AOC; antibody delivery; bioconjugation; protein conjugate vaccines; protein delivery; protein therapeutics; protein–polymer conjugates.
Conflict of interest statement
All authors were employees of Genentech, Inc. at the time of publication.
Figures




References
-
- FDA Approved PEGylated Drugs Up To 2022. [(accessed on 12 December 2022)]. Available online: https://www.biochempeg.com/article/58.html.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

