Screening of Fenofibrate-Simvastatin Solid Dispersions in the Development of Fixed-Dose Formulations for the Treatment of Lipid Disorders
- PMID: 36839925
- PMCID: PMC9962408
- DOI: 10.3390/pharmaceutics15020603
Screening of Fenofibrate-Simvastatin Solid Dispersions in the Development of Fixed-Dose Formulations for the Treatment of Lipid Disorders
Abstract
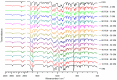
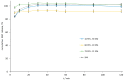
The combination of statins and fibrates in the treatment of lipid abnormalities effectively regulates individual lipid fraction levels. In this study, the screening and assessment of the physicochemical properties of simvastatin-fenofibrate solid dispersions were performed. Fenofibrate and simvastatin were processed using the kneading method in different weight ratios, and the resulting solid dispersions were assessed using differential scanning calorimetry (DSC), X-ray powder diffractometry (XRPD), Fourier-transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), contact angle, as well as dissolution tests. The obtained results confirmed the formation of a simple eutectic phase diagram, with a eutectic point containing 79 wt% fenofibrate and 21 wt% simvastatin, lack of chemical interactions between the ingredients, and simvastatin impact on improving fenofibrate dissolution profile, due to the formation of crystalline solid dispersions by the kneading method.
Keywords: dissolution improvement; dyslipidemia; eutectic; fenofibrate; fixed-dose combination; simvastatin; solid dispersion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












References
-
- Godman B., McCabe H., Leong T.D., Mueller D., Martin A.P., Hoxha I., Mwita J.C., Rwegerera G.M., Massele A., de Oliveira Costa J. Fixed dose drug combinations—Are they pharmacoeconomically sound? Findings and implications especially for lower- and middle-income countries. Expert Rev. Pharmacoecon. Outcomes Res. 2020;20:1–26. doi: 10.1080/14737167.2020.1734456. - DOI - PubMed
-
- Chen C.-Y., Lee C.-W., Chien S.-C., Su M.-I., Lin S.-I., Cheng C.-W., Hung T.-C., Yeh H.-I. Dyslipidemia management for elderly people with metabolic syndrome: A mini-review. Int. J. Gerontol. 2018;12:7–11. doi: 10.1016/j.ijge.2017.07.001. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

