The Ability of Some Polysaccharides to Disaggregate Lysozyme Amyloid Fibrils and Renature the Protein
- PMID: 36839946
- PMCID: PMC9962556
- DOI: 10.3390/pharmaceutics15020624
The Ability of Some Polysaccharides to Disaggregate Lysozyme Amyloid Fibrils and Renature the Protein
Abstract
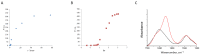
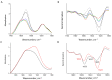
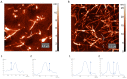
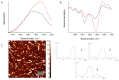
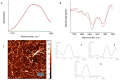
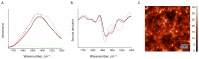
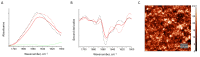
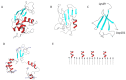
The deposition of proteins in the form of insoluble amyloid fibril aggregates is linked to a range of diseases. The supramolecular architecture of such deposits is governed by the propagation of β-strands in the direction of protofilament growth. In the present study, we analyze the structural changes of hen egg-white lysozyme fibrils upon their interactions with a range of polysaccharides, using AFM and FTIR spectroscopy. Linear anionic polysaccharides, such as κ-carrageenan and sodium alginate, are shown to be capable to disaggregate protofilaments with eventual protein renaturation. The results help to understand the mechanism of amyloid disaggregation and create a platform for both the development of new therapeutic agents for amyloidose treatment, and the design of novel functional protein-polysaccharide complex-based nanomaterials.
Keywords: alginate; amyloid fibrils; atomic force microscopy; carrageenan; chitosan; disaggregation; galactan; infrared spectroscopy; lysozyme; polysaccharides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Boersema P.J., Melnik A., Hazenberg B.P.C., Rezeli M., Marko-Varga G., Kamiie J., Portelius E., Blennow K., Zubarev R.A., Polymenidou M., et al. Biology/Disease-Driven Initiative on Protein-Aggregation Diseases of the Human Proteome Project: Goals and Progress to Date. J. Proteome Res. 2018;17:4072–4084. doi: 10.1021/acs.jproteome.8b00401. - DOI - PubMed
-
- Debnath K., Sarkar A.K., Jana N.R., Jana N.R. Inhibiting Protein Aggregation by Small Molecule-Based Colloidal Nanoparticles. Acc. Mater. Res. 2022;3:54–66. doi: 10.1021/accountsmr.1c00193. - DOI
-
- Wu L., Velander P., Brown A.M., Wang Y., Liu D., Bevan D.R., Zhang S., Xu B. Rosmarinic Acid Potently Detoxifies Amylin Amyloid and Ameliorates Diabetic Pathology in a Transgenic Rat Model of Type 2 Diabetes. ACS Pharmacol. Transl. Sci. 2021;4:1322–1337. doi: 10.1021/acsptsci.1c00028. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

