Anti- Trypanosoma cruzi Properties of Sesquiterpene Lactones Isolated from Stevia spp.: In Vitro and In Silico Studies
- PMID: 36839969
- PMCID: PMC9961625
- DOI: 10.3390/pharmaceutics15020647
Anti- Trypanosoma cruzi Properties of Sesquiterpene Lactones Isolated from Stevia spp.: In Vitro and In Silico Studies
Abstract
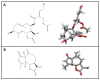
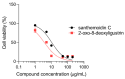
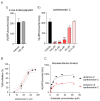
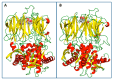
Stevia species (Asteraceae) have been a rich source of terpenoid compounds, mainly sesquiterpene lactones, several of which show antiprotozoal activity. In the search for new trypanocidal compounds, S. satureiifolia var. satureiifolia and S. alpina were studied. Two sesquiterpene lactones, santhemoidin C and 2-oxo-8-deoxyligustrin, respectively, were isolated. These compounds were assessed in vitro against Trypanosoma cruzi stages, showing IC50 values of 11.80 and 4.98 on epimastigotes, 56.08 and 26.19 on trypomastigotes and 4.88 and 20.20 µM on amastigotes, respectively. Cytotoxicity was evaluated on Vero cells by the MTT assay. The effect of the compounds on trypanothyone reductase (TcTR), Trans-sialidase (TcTS) and the prolyl oligopeptidase of 80 kDa (Tc80) as potential molecular targets of T. cruzi was investigated. Santhemoidin C inhibited oligopeptidase activity when tested against recombinant Tc80 using a fluorometric assay, reaching an IC50 of 34.9 µM. Molecular docking was performed to study the interaction between santhemoidin C and the Tc80 protein, reaching high docking energy levels. Plasma membrane shedding and cytoplasmic vacuoles, resembling autophagosomes, were detected by transmission microscopy in parasites treated with santhemoidin C. Based on these results, santhemoidin C represents a promising candidate for further studies in the search for new molecules for the development of trypanocidal drugs.
Keywords: 2-oxo-8-deoxyligustrin; Stevia alpina; Stevia satureiifolia var satureiifolia; molecular targets; prolyl oligopeptidase; santhemoidin C; sesquiterpene lactones; trypanocidal activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Trypanocidal and leishmanicidal activities of flavonoids isolated from Stevia satureiifolia var. satureiifolia.Pharm Biol. 2016 Oct;54(10):2188-95. doi: 10.3109/13880209.2016.1150304. Epub 2016 Mar 17. Pharm Biol. 2016. PMID: 26983579
-
Antiprotozoal Compounds from Urolepis hecatantha (Asteraceae).Evid Based Complement Alternat Med. 2021 Feb 11;2021:6622894. doi: 10.1155/2021/6622894. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 33628303 Free PMC article.
-
Germacranolide-type sesquiterpene lactones from Smallanthus sonchifolius with promising activity against Leishmania mexicana and Trypanosoma cruzi.Parasit Vectors. 2017 Nov 13;10(1):567. doi: 10.1186/s13071-017-2509-6. Parasit Vectors. 2017. PMID: 29132413 Free PMC article.
-
In silico study of structural and geometrical requirements of natural sesquiterpene lactones with trypanocidal activity.Mini Rev Med Chem. 2013 Aug;13(10):1407-14. doi: 10.2174/13895575113139990066. Mini Rev Med Chem. 2013. PMID: 23815577 Review.
-
Proteinases of Trypanosoma cruzi: patential targets for the chemotherapy of Changas desease.Curr Top Med Chem. 2002 Nov;2(11):1261-71. doi: 10.2174/1568026023392995. Curr Top Med Chem. 2002. PMID: 12171584 Review.
Cited by
-
Plant Extracts and Phytochemicals from the Asteraceae Family with Antiviral Properties.Molecules. 2024 Feb 9;29(4):814. doi: 10.3390/molecules29040814. Molecules. 2024. PMID: 38398567 Free PMC article.
-
Extracts and Terpenoids from Stevia Species as Potential Anthelmintics for Neglected Tropical Diseases Caused by Cestode Parasites.Molecules. 2024 Sep 18;29(18):4430. doi: 10.3390/molecules29184430. Molecules. 2024. PMID: 39339424 Free PMC article.
-
MolPredictX: A Pioneer Mobile App Version for Online Biological Activity Predictions by Machine Learning Models.Methods Mol Biol. 2025;2834:351-371. doi: 10.1007/978-1-0716-4003-6_17. Methods Mol Biol. 2025. PMID: 39312174
-
Trypanocidal and Anti-Inflammatory Effects of Three ent-Kaurane Diterpenoids from Gymnocoronis spilanthoides var. subcordata (Asteraceae).Pharmaceutics. 2024 Mar 18;16(3):415. doi: 10.3390/pharmaceutics16030415. Pharmaceutics. 2024. PMID: 38543309 Free PMC article.
References
-
- Rodríguez-Cravero J.F., Gutiérrez D.G., Katinas L., Grossi M.A., Bonifacino J.M., Marchesi E. A Revision and Morphological Analysis of the Uruguayan Species of Stevia (Compositae, Eupatorieae) Rodriguésia. 2019;70 doi: 10.1590/2175-7860201970078. - DOI
-
- Soejarto D.D., Compadre C.M., Kinghorn A.D. Ethnobotanical Notes on Stevia. Bot. Mus. Leafl. Harv. Univ. 1983;29:1–25. doi: 10.5962/p.168652. - DOI
-
- Hernandez L., Catalan C., Joseph-Nathan P. The Chemistry of the Genus Stevia (Asteraceae) Rev. De La Acad. Colomb. De Cienc. Exactas Físicas Y Nat. 1998;22:229–279.
-
- Sülsen V., Martino V. Sesquiterpene Lactones: Advances in Their Chemistry and Biological Aspects. Springer; Cham, Switzerland: 2018.
Grants and funding
- 20020170100316BA/University of Buenos Aires
- 20020130100788BA/University of Buenos Aires
- 11220150100158CO/National Scientific and Technical Research Council
- PUE-0017CO-2016/National Scientific and Technical Research Council
- PICT 2020--SERIEA-03061/Agencia Nacional de Promoción Científica y Tecnológica
LinkOut - more resources
Full Text Sources
Miscellaneous

