Efficacy and Safety of Inhaled Ethanol in Early-Stage SARS-CoV-2 Infection in Older Adults: A Phase II Randomized Clinical Trial
- PMID: 36839987
- PMCID: PMC9966500
- DOI: 10.3390/pharmaceutics15020667
Efficacy and Safety of Inhaled Ethanol in Early-Stage SARS-CoV-2 Infection in Older Adults: A Phase II Randomized Clinical Trial
Abstract
Background: Inhaled ethanol in the early stages of SARS-CoV-2 infection may reduce the viral load, decreasing progression and improving prognosis. The ALCOVID-19 trial was designed to study the efficacy and safety of inhaled ethanol in older adults at initial phases of infection.
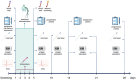
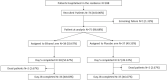
Methods: Randomized, triple-blind, placebo-controlled phase II clinical trial. Experimental group (n = 38) inhaled 65° ethanol through an oxygen flow, while in the control group (n = 37), water for injection was used. General endpoint was to evaluate disease progression according to the modified World Health Organization (WHO) Clinical Progression Scale. Specific effectiveness endpoints were body temperature, oxygen saturation, viral load assessed by cycle threshold (Ct) on real-time polymerase chain reaction (RT-PCR), analytical biomarkers and use of antibiotics or corticosteroids. Specific safety outcomes were the absence of ethanol in plasma, electrographic, analytical, or respiratory alterations.
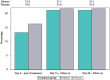
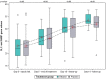
Results: In the intention-to-treat population, no differences were found regarding disease progression. Mean Ct values increased over time in both groups, being numerically higher in the ethanol group, reaching a value above 33 only in the ethanol group on day 14, a value above which patients are considered non-infective. No differences were found in the other specific effectiveness endpoints. Inhaled ethanol was proven to be safe as no plasma ethanol was detected, and there were no electrocardiographic, analytical, or respiratory alterations.
Conclusions: The efficacy of inhaled ethanol in terms of the progression of SARS-CoV-2 infection was not demonstrated in the present trial. However, it is positioned as a safe treatment for elderly patients with early-stage COVID-19.
Keywords: COVID-19; SARS-CoV-2; elderly; inhaled ethanol; randomized controlled trial.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Testing the efficacy and safety of BIO101, for the prevention of respiratory deterioration, in patients with COVID-19 pneumonia (COVA study): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Jan 11;22(1):42. doi: 10.1186/s13063-020-04998-5. Trials. 2021. PMID: 33430924 Free PMC article.
-
Reconvalescent plasma/camostat mesylate in early SARS-CoV-2 Q-PCR positive high-risk individuals (RES-Q-HR): a structured summary of a study protocol for a randomized controlled trial.Trials. 2021 May 17;22(1):343. doi: 10.1186/s13063-021-05181-0. Trials. 2021. PMID: 34001215 Free PMC article.
-
Randomized clinical trial to compare the efficacy of ivermectin versus placebo to negativize nasopharyngeal PCR in patients with early COVID-19 in Peru (SAINT-Peru): a structured summary of a study protocol for randomized controlled trial.Trials. 2021 Apr 9;22(1):262. doi: 10.1186/s13063-021-05236-2. Trials. 2021. PMID: 33836826 Free PMC article.
-
The SARS-CoV-2 Ivermectin Navarra-ISGlobal Trial (SAINT) to Evaluate the Potential of Ivermectin to Reduce COVID-19 Transmission in low risk, non-severe COVID-19 patients in the first 48 hours after symptoms onset: A structured summary of a study protocol for a randomized control pilot trial.Trials. 2020 Jun 8;21(1):498. doi: 10.1186/s13063-020-04421-z. Trials. 2020. PMID: 32513289 Free PMC article.
Cited by
-
Ethanol Inhalation for Respiratory Infections due to Enveloped Viruses.Infect Dis Ther. 2025 Jun;14(6):1143-1156. doi: 10.1007/s40121-025-01157-8. Epub 2025 Apr 17. Infect Dis Ther. 2025. PMID: 40246793 Free PMC article. Review.
-
Positive Correlation Between Heavy Alcoholic Drinking and SARS-Cov-2 Non-Infection Rate.Cureus. 2023 Jun 8;15(6):e40130. doi: 10.7759/cureus.40130. eCollection 2023 Jun. Cureus. 2023. PMID: 37304380 Free PMC article.
References
-
- de Labry-Lima A.O., Saez-de la Fuente J., Martin L.A.-K., Alegre-Del Rey E.J., García-Cabrera E., Sierra-Sánchez J.F. Factors Associated with Mortality in Patients Hospitalized for COVID-19 in Spain. Data from the RERFAR Registry. Farm. Hosp. 2022;46:57–71. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

