Structure-Activity Relationship Studies of 9-Alkylamino-1,2,3,4-tetrahydroacridines against Leishmania (Leishmania) infantum Promastigotes
- PMID: 36839991
- PMCID: PMC9965875
- DOI: 10.3390/pharmaceutics15020669
Structure-Activity Relationship Studies of 9-Alkylamino-1,2,3,4-tetrahydroacridines against Leishmania (Leishmania) infantum Promastigotes
Abstract
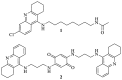
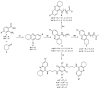
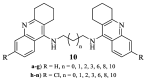
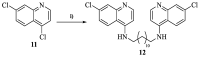
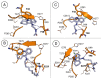
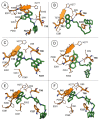
Leishmaniasis is one of the most neglected diseases in modern times, mainly affecting people from developing countries of the tropics, subtropics and the Mediterranean basin, with approximately 350 million people considered at risk of developing this disease. The incidence of human leishmaniasis has increased over the past decades due to failing prevention and therapeutic measures-there are no vaccines and chemotherapy, which is problematic. Acridine derivatives constitute an interesting group of nitrogen-containing heterocyclic compounds associated with numerous bioactivities, with emphasis to their antileishmanial potential. The present work builds on computational studies focusing on a specific enzyme of the parasite, S-adenosylmethionine decarboxylase (AdoMet DC), with several 1,2,3,4-tetrahydro-acridines emerging as potential inhibitors, evidencing this scaffold as a promising building block for novel antileishmanial pharmaceuticals. Thus, several 1,2,3,4-tetrahydroacridine derivatives have been synthesized, their activity against Leishmania (Leishmania) infantum promastigotes evaluated and a structure-activity relationship (SAR) study was developed based on the results obtained. Even though the majority of the 1,2,3,4-tetrahydroacridines evaluated presented high levels of toxicity, the structural information gathered in this work allowed its application with another scaffold (quinoline), leading to the obtention of N1,N12-bis(7-chloroquinolin-4-yl)dodecane-1,12-diamine (12) as a promising novel antileishmanial agent (IC50 = 0.60 ± 0.11 μM, EC50 = 11.69 ± 3.96 μM and TI = 19.48).
Keywords: 1,2,3,4-tetrahydroacridine; AdoMet DC; Leishmania; macrophages; microwave-assisted synthesis; molecular docking; promastigotes; quinoline; virtual screening.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- World Health Organization (WHO) First WHO Report on Neglected Tropical Diseases: Working to Overcome the Global Impact of Neglected Tropical Diseases. WHO; Geneva, Switzerland: 2010.
-
- World Health Organization (WHO) The Control of Leishmaniases. Volume 949 WHO; Geneva, Switzerland: 2010.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

