Metallodrugs against Breast Cancer: Combining the Tamoxifen Vector with Platinum(II) and Palladium(II) Complexes
- PMID: 36840003
- PMCID: PMC9959148
- DOI: 10.3390/pharmaceutics15020682
Metallodrugs against Breast Cancer: Combining the Tamoxifen Vector with Platinum(II) and Palladium(II) Complexes
Erratum in
-
Correction: Kazimir et al. Metallodrugs against Breast Cancer: Combining the Tamoxifen Vector with Platinum(II) and Palladium(II) Complexes. Pharmaceutics 2023, 15, 682.Pharmaceutics. 2023 Jun 19;15(6):1769. doi: 10.3390/pharmaceutics15061769. Pharmaceutics. 2023. PMID: 37376228 Free PMC article.
Abstract
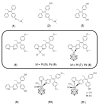
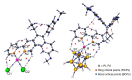
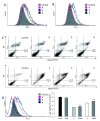
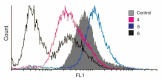
The luminal A-subtype of breast cancer, where the oestrogen receptor α (ERα) is overexpressed, is the most frequent one. The prodrug tamoxifen (1) is the clinically used agent, inhibiting the ERα activity via the formation of several active metabolites, such as 4-hydroxytamoxifen (2) or 4,4'-dihydroxytamoxifen (3). In this study, we present the tamoxifen derivative 4-[1,1-bis(4-methoxyphenyl)but-1-en-2-yl]-2,2'-bipyridine (4), which was combined with platinum or palladium dichloride, the former a well-known scaffold in anticancer treatment, to give [PtCl2(4-κ2N,N')] (5) or [PdCl2(4-κ2N,N'] (6). To prevent fast exchange of weakly coordinating chlorido ligands in aqueous solution, a bulky, highly stable and hydrophobic nido-carborate(-2) ([C2B9H11]2-) was incorporated. The resulting complexes [3-(4-κ2N,N')-3,1,2-PtC2B9H11] (7) and [3-(4-κ2N,N')-3,1,2-PdC2B9H11] (8) exhibit a dramatic change in electronic and biological properties compared to 5 and 6. Thus, 8 is highly selective for triple-negative MDA-MB-231 cells (IC50 = 3.7 μM, MTT test), while 7 is completely inactive against this cell line. The observed cytotoxicity of compounds 4-6 and 8 against this triple-negative cell line suggests off-target mechanisms rather than only ERα inhibition, for which these compounds were originally designed. Spectroscopic properties and electronic structures of the metal complexes were investigated for possible explanations of the biological activities.
Keywords: breast cancer; cytotoxicity; oxidative stress; palladacarboranes; palladium dichloride; platinacarboranes; platinum dichloride; tamoxifen derivative.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Sisci D., Maris P., Grazia Cesario M., Anselmo W., Coroniti R., Elvi Trombino G., Romeo F., Ferraro A., Lanzino M., Aquila S., et al. The Estrogen Receptor α Is the Key Regulator of the Bifunctional Role of FoxO3a Transcription Factor in Breast Cancer Motility and Invasiveness. Cell Cycle. 2013;12:3405–3420. doi: 10.4161/cc.26421. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

