Coconut Callus Initiation for Cell Suspension Culture
- PMID: 36840315
- PMCID: PMC9961714
- DOI: 10.3390/plants12040968
Coconut Callus Initiation for Cell Suspension Culture
Abstract
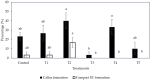
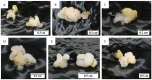
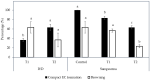
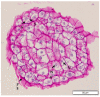
The development of a cell suspension culture system for the scaling up of coconut embryogenic callus (EC) production would drastically improve efforts to achieve the large-scale production of high-quality clonal plantlets. To date, the hard nature of coconut EC appeared to be the main constraint for developing cell suspension cultures. Hence, this study attempted to acquire friable EC through the following approaches: The manipulation of (1) medium type and subculture frequency, (2) a reduced 2,4-dichlorophenoxy acetic acid concentration during subculture, (3) the nitrate level and the ammonium-to-nitrate ratio, and the addition of amino acid mixture, (4) the addition of L-proline, and (5) the reduction of medium nutrients. Unfortunately, none of these culture conditions produced friable coconut EC. Even though friable EC was not achieved via these approaches, some of the conditions were found to influence the formation of compact EC, therefore these results are important for further studies focused on somatic embryogenesis in coconut and other species.
Keywords: Cocos nucifera L.; callus; cell suspension culture; coconut; embryogenic callus; somatic embryogenesis; tissue culture.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures







Similar articles
-
Addition of ionophore A23187 increases the efficiency of Cocos nucifera somatic embryogenesis.3 Biotech. 2018 Aug;8(8):366. doi: 10.1007/s13205-018-1392-y. Epub 2018 Aug 10. 3 Biotech. 2018. PMID: 30105191 Free PMC article.
-
Abscisic acid induced somatic embryogenesis in immature embryo explants of coconut (Cocos nucifera L.).Plant Sci. 2000 Feb 21;151(2):193-198. doi: 10.1016/s0168-9452(99)00218-6. Plant Sci. 2000. PMID: 10808075
-
Dynamic changes in the expression pattern of miRNAs and associated target genes during coconut somatic embryogenesis.Planta. 2020 Mar 12;251(4):79. doi: 10.1007/s00425-020-03368-4. Planta. 2020. PMID: 32166498
-
Tissue culture and associated biotechnological interventions for the improvement of coconut (Cocos nucifera L.): a review.Planta. 2015 Nov;242(5):1059-76. doi: 10.1007/s00425-015-2362-9. Epub 2015 Jul 19. Planta. 2015. PMID: 26189000 Review.
-
Cloning Coconut via Somatic Embryogenesis: A Review of the Current Status and Future Prospects.Plants (Basel). 2021 Sep 29;10(10):2050. doi: 10.3390/plants10102050. Plants (Basel). 2021. PMID: 34685859 Free PMC article. Review.
Cited by
-
Phytosulfokine contributes to suspension culture of Cunninghamia lanceolata through its impact on redox homeostasis.BMC Plant Biol. 2023 Oct 9;23(1):480. doi: 10.1186/s12870-023-04496-1. BMC Plant Biol. 2023. PMID: 37814230 Free PMC article.
-
Comparative analysis of endophytic bacterial localization and microbiome diversity in plant varieties under varied growth conditions through microscopic imaging and sequencing techniques.Front Microbiol. 2025 May 16;16:1568209. doi: 10.3389/fmicb.2025.1568209. eCollection 2025. Front Microbiol. 2025. PMID: 40454365 Free PMC article.
-
Callus Culture System from Lonicera japonica Thunb Anthers: Light Quality Effects on Callus Quality Evaluation.Int J Mol Sci. 2025 Mar 6;26(5):2351. doi: 10.3390/ijms26052351. Int J Mol Sci. 2025. PMID: 40076969 Free PMC article.
References
-
- Pérez-Núñez M.T., Chan J.L., Sáenz L., González T., Verdeil J.L., Oropeza C. Improved somatic embryogenesis from Cocos nucifera (L.) plumule explants. Vitr. Cell. Dev. Biol. Plant. 2006;42:37–43. doi: 10.1079/IVP2005722. - DOI
-
- Salum U., Foale M., Biddle J., Bazrafshan A., Adkins S. Towards the sustainability of the “tree of life”: An introduction. In: Adkins S., Foale M., Bourdeix R., Nguyen Q., Biddle J., editors. Coconut Biotechnology: Towards the Sustainability of the ‘Tree of Life’. Springer International Publishing; Cham, Switzerland: 2020. pp. 1–15. - DOI
-
- Ascough G.D., Fennell C.W. The regulation of plant growth and development in liquid culture. S. Afr. J. Bot. 2004;70:181–190. doi: 10.1016/S0254-6299(15)30234-9. - DOI
-
- Teixeira J.B., Söndahl M.R., Nakamura T., Kirby E.G. Establishment of oil palm cell suspensions and plant regeneration. Plant Cell Tissue Organ Cult. 1995;40:105–111. doi: 10.1007/BF00037662. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

