OxPAPC stabilizes liquid-ordered domains in biomimetic membranes
- PMID: 36840353
- PMCID: PMC10111260
- DOI: 10.1016/j.bpj.2023.02.024
OxPAPC stabilizes liquid-ordered domains in biomimetic membranes
Abstract
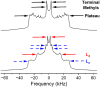
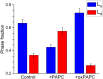
Long-chain polyunsaturated fatty acids (PUFAs) are prone to nonenzymatic oxidation in response to differing environmental stressors and endogenous cellular sources. There is increasing evidence that phospholipids containing oxidized PUFA acyl chains control the inflammatory response. However, the underlying mechanism(s) of action by which oxidized PUFAs exert their functional effects remain unclear. Herein, we tested the hypothesis that replacement of 1-palmitoyl-2-arachidonyl-phosphatidylcholine (PAPC) with oxidized 1-palmitoyl-2-arachidonyl-phosphatidylcholine (oxPAPC) regulates membrane architecture. Specifically, with solid-state 2H NMR of biomimetic membranes, we investigated how substituting oxPAPC for PAPC modulates the molecular organization of liquid-ordered (Lo) domains. 2H NMR spectra for bilayer mixtures of 1,2-dipalmitoylphosphatidylcholine-d62 (an analog of DPPC deuterated throughout sn-1 and -2 chains) and cholesterol to which PAPC or oxPAPC was added revealed that replacing PAPC with oxPAPC disrupted molecular organization, indicating that oxPAPC does not mix favorably in a tightly packed Lo phase. Furthermore, unlike PAPC, adding oxPAPC stabilized 1,2-dipalmitoylphosphatidylcholine-d6-rich/cholesterol-rich Lo domains formed in mixtures with 1,2-dioleoylphosphatidylcholine while decreasing the molecular order within 1,2-dioleoylphosphatidylcholine-rich liquid-disordered regions of the membrane. Collectively, these results suggest a mechanism in which oxPAPC stabilizes Lo domains-by disordering the surrounding liquid-disordered region. Changes in the structure, and thereby functionality, of Lo domains may underly regulation of plasma membrane-based inflammatory signaling by oxPAPC.
Copyright © 2023 Biophysical Society. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

