In Situ-Formed Fibrin Hydrogel Scaffold Loaded With Human Umbilical Cord Mesenchymal Stem Cells Promotes Skin Wound Healing
- PMID: 36840468
- PMCID: PMC9969468
- DOI: 10.1177/09636897231156215
In Situ-Formed Fibrin Hydrogel Scaffold Loaded With Human Umbilical Cord Mesenchymal Stem Cells Promotes Skin Wound Healing
Abstract
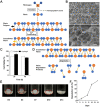
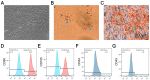
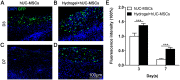
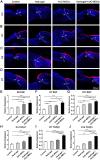
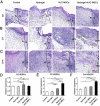
Healing of full-thickness skin wounds remains a major challenge. Recently, human umbilical cord mesenchymal stem cells (hUC-MSCs) were shown to possess an extraordinary potential to promote skin repair in clinical settings. However, their low survival rate after transplantation limits their therapeutic efficiency in treating full-thickness skin wounds. Hydrogels are considered an ideal cell transplantation vector owing to their three-dimensional mesh structure, good biosafety, and biodegradation. The objective of this study was to investigate the skin wound healing effect of a fibrin hydrogel scaffold loaded with hUC-MSCs. We found that the fibrin hydrogel had a three-dimensional mesh structure and low cytotoxicity and could prolong the time of cell survival in the peri-wound area. The number of green fluorescent protein (GFP)-labeled hUC-MSCs was higher in the full-thickness skin wound of mice treated with hydrogel-hUC-MSCs than those of mice treated with cell monotherapy. In addition, the combination therapy between the hydrogel and hUC-MSCs speed up wound closure, its wound healing rate was significantly higher than those of phosphate-buffered saline (PBS) therapy, hydrogel monotherapy, and hUC-MSCs monotherapy. Furthermore, the results showed that the combination therapy between hydrogel and hUC-MSCs increased keratin 10 and keratin 14 immunofluorescence staining, and upregulated the relative gene expressions of epidermal growth factor (EGF), transforming growth factor-β1 (TGF-β1), and vascular endothelial growth factor A (VEGFA), promoting epithelial regeneration and angiogenesis. In conclusion, the fibrin hydrogel scaffold provides a relatively stable sterile environment for cell adhesion, proliferation, and migration, and prolongs cell survival at the wound site. The hydrogel-hUC-MSCs combination therapy promotes wound closure, re-epithelialization, and neovascularization. It exhibits a remarkable therapeutic effect, being more effective than the monotherapy with hUC-MSCs or hydrogel.
Keywords: fibrin; human umbilical cord mesenchymal stem cells; hydrogel; skin wound healing.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

References
-
- Zheng Z, Bian S, Li Z, Zhang Z, Liu Y, Zhai X, Pan H, Zhao X. Catechol modified quaternized chitosan enhanced wet adhesive and antibacterial properties of injectable thermo-sensitive hydrogel for wound healing. Carbohydr Polym. 2020;249:116826. - PubMed
-
- Lei Z, Singh G, Min Z, Shixuan C, Xu K, Pengcheng X, Xueer W, Yinghua C, Lu Z, Lin Z. Bone marrow-derived mesenchymal stem cells laden novel thermo-sensitive hydrogel for the management of severe skin wound healing. Mater Sci Eng C Mater Biol Appl. 2018;90:159–67. - PubMed
-
- Gurtner G, Werner S, Barrandon Y, Longaker M. Wound repair and regeneration. Nature. 2008;453(7193):314–21. - PubMed
-
- Walter MNM, Wright KT, Fuller HR, MacNeil S, Johnson WEB. Mesenchymal stem cell-conditioned medium accelerates skin wound healing: an in vitro study of fibroblast and keratinocyte scratch assays. Exp Cell Res. 2010;316(7):1271–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

