Chemogenetic inhibition of a monosynaptic projection from the basolateral amygdala to the ventral hippocampus selectively reduces appetitive, but not consummatory, alcohol drinking-related behaviours
- PMID: 36840503
- PMCID: PMC10931538
- DOI: 10.1111/ejn.15944
Chemogenetic inhibition of a monosynaptic projection from the basolateral amygdala to the ventral hippocampus selectively reduces appetitive, but not consummatory, alcohol drinking-related behaviours
Abstract
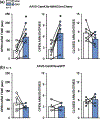
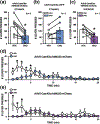
Alcohol use disorder (AUD) and anxiety/stressor disorders frequently co-occur and this dual diagnosis represents a major health and economic problem worldwide. The basolateral amygdala (BLA) is a key brain region that is known to contribute to the aetiology of both disorders. Although many studies have implicated BLA hyperexcitability in the pathogenesis of AUD and comorbid conditions, relatively little is known about the specific efferent projections from this brain region that contribute to these disorders. Recent optogenetic studies have shown that the BLA sends a strong monosynaptic excitatory projection to the ventral hippocampus (vHC) and that this circuit modulates anxiety- and fear-related behaviours. However, it is not known if this pathway influences alcohol drinking-related behaviours. Here, we employed a rodent operant self-administration regimen that procedurally separates appetitive (e.g. seeking) and consummatory (e.g., drinking) behaviours, chemogenetics and brain region-specific microinjections, to determine if BLA-vHC circuitry influences alcohol and sucrose drinking-related measures. We first confirmed prior optogenetic findings that silencing this circuit reduced anxiety-like behaviours on the elevated plus maze. We then demonstrated that inhibiting the BLA-vHC pathway significantly reduced appetitive drinking-related behaviours for both alcohol and sucrose while having no effect on consummatory measures. Taken together, these findings provide the first indication that the BLA-vHC circuit may regulate appetitive reward seeking directed at alcohol and natural rewards and add to a growing body of evidence suggesting that dysregulation of this pathway may contribute to the pathophysiology of AUD and anxiety/stressor-related disorders.
Keywords: alcohol addiction; anxiety; motivation; seeking; ventral subiculum.
© 2023 The Authors. European Journal of Neuroscience published by Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
The authors declare no competing financial interests.
Figures



References
-
- Bach EC, Morgan JW, Ewin SE, Barth SH, Raab-Graham K, & Weiner JL (2021). Chronic ethanol exposure leads to a negative affective state in female rats that is accompanied by a paradoxical decrease in ventral hippocampus excitability. Frontiers in Neuroscience, 15, 669075. 10.3389/fnins.2021.669075 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

