Development of a selection assay for small guide RNAs that drive efficient site-directed RNA editing
- PMID: 36840708
- PMCID: PMC10123091
- DOI: 10.1093/nar/gkad098
Development of a selection assay for small guide RNAs that drive efficient site-directed RNA editing
Abstract
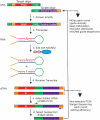
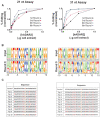
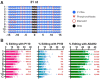
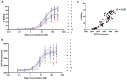
A major challenge confronting the clinical application of site-directed RNA editing (SDRE) is the design of small guide RNAs (gRNAs) that can drive efficient editing. Although many gRNA designs have effectively recruited endogenous Adenosine Deaminases that Act on RNA (ADARs), most of them exceed the size of currently FDA-approved antisense oligos. We developed an unbiased in vitro selection assay to identify short gRNAs that promote superior RNA editing of a premature termination codon. The selection assay relies on hairpin substrates in which the target sequence is linked to partially randomized gRNAs in the same molecule, so that gRNA sequences that promote editing can be identified by sequencing. These RNA substrates were incubated in vitro with ADAR2 and the edited products were selected using amplification refractory mutation system PCR and used to regenerate the substrates for a new round of selection. After nine repetitions, hairpins which drove superior editing were identified. When gRNAs of these hairpins were delivered in trans, eight of the top ten short gRNAs drove superior editing both in vitro and in cellula. These results show that efficient small gRNAs can be selected using our approach, an important advancement for the clinical application of SDRE.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

