Comparison of Real-World Costs, Healthcare Resource Utilization, and Comorbidity-Related Costs Between Ixekizumab and Secukinumab Among Biologic-Experienced Patients with Psoriasis Over 18 Months in the USA
- PMID: 36840815
- PMCID: PMC10011324
- DOI: 10.1007/s40261-022-01240-9
Comparison of Real-World Costs, Healthcare Resource Utilization, and Comorbidity-Related Costs Between Ixekizumab and Secukinumab Among Biologic-Experienced Patients with Psoriasis Over 18 Months in the USA
Abstract
Background and objective: Data on real-world healthcare costs for ixekizumab (IXE) and secukinumab (SEC) in biologic-experienced patients with psoriasis are limited. This study compared real-world costs and healthcare resource utilization between IXE and SEC in biologic-experienced patients with psoriasis over an 18-month follow-up period in the USA.
Methods: Adult patients with a diagnosis of psoriasis between 1 March, 2015 and 31 October, 2019 were identified using health insurance claims data from IBM Watson Health MarketScan®. The index date was the date of the first IXE or SEC claim. Biologic-experienced patients with one or more pre-period claims for biologic drugs were identified. Inverse probability of treatment weighting was used to reduce cohort imbalances. All-cause and psoriasis-related direct healthcare costs along with index drug costs were estimated during the follow-up and reported as per patient per month. Discount factors published by the Institute for Clinical and Economic Review were applied to psoriasis-related biologics to adjust pharmacy costs.
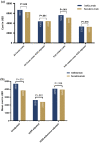
Results: A total of 411 IXE and 780 SEC users were included. After weighting, all-cause inpatient admissions were similar between IXE (9.5%) and SEC users (10.3%). Weighted, mean ± standard deviation per patient per month all-cause healthcare costs were higher in IXE users ($6670 ± $2910) than in SEC users ($6239 ± $3903; p = 0.049). Psoriasis-related and monthly index drug costs were higher in IXE users ($5609 ± $2009; p < 0.001 and $4688 ± $1994; p < 0.001, respectively) than in SEC users ($5095 ± $2291 and $3853 ± $1977, respectively). After Institute for Clinical and Economic Review adjustment, mean per patient per month all-cause ($4363 ± $2576 vs $4398 ± $3517) and psoriasis-related costs ($3302 ± $1264 vs $3253 ± $1504) were similar between the groups. Institute for Clinical and Economic Review- and adherence-adjusted mean per patient per month index drug costs were similar between IXE and SEC users (p = 0.339).
Conclusions: Institute for Clinical and Economic Review-adjusted all-cause and psoriasis-related costs were comparable between IXE and SEC users among biologic-experienced patients over an 18-month follow-up period.
© 2023. The Author(s).
Conflict of interest statement
Andrew Blauvelt has served as a scientific adviser and/or clinical study investigator for AbbVie, Abcentra, Aligos, Almirall, Amgen, Arcutis, Arena, Aslan, Athenex, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant, EcoR1, Eli Lilly and Company, Evommune, Forte, Galderma, Incyte, Janssen, Landos, Leo, Novartis, Pfizer, Rapt, Regeneron, Sanofi Genzyme, Sun Pharma, UCB Pharma, and Vibliome. Nianwen Shi, Carolyn R. Lew, and Nicole M. Zimmerman are employees of IBM Watson Health who were compensated by Eli Lilly and Company for conducting this research. Najwa Somani, Scott A. Kern, Russel Burge, Terri Ridenour, Baojin Zhu, and Mwangi Murage are full-time employees and stockholders of Eli Lilly and Company.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

