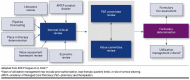
Methodology for conducting a comprehensive product review in managed care
- PMID: 36840955
- PMCID: PMC10388012
- DOI: 10.18553/jmcp.2023.29.3.237
Methodology for conducting a comprehensive product review in managed care
Abstract
The high degree of complexity of the product-review process and differences in procedures between organizations have resulted in a need for best practices and an overall product-review process to create efficiencies for health care decision makers. In an effort to streamline product-review concepts, this article outlines the different components of the review process, including clinical and economic review, formulary placement determination, and evaluation of alternatives within a drug class. The article also details opportunities for the near future, as technology continues to advance and alignment between medical and pharmacy benefits is desired. DISCLOSURES: Drs Linnerooth, Penley, Ha, and Craven report employment with Xcenda, which provided funding for the manuscript. Drs Sauvageau and Hydery report employment Xcenda, which provided funding for the manuscript, and stock holdings with AmerisourceBergen. Dr Feeney reports support for attending meetings and/or travel provided by Highmark, Inc. Dr Thomas reports receipt of consulting fees from ActiveRADAR, board member roles with ActiveRADAR and RoundtableRx, an adjunct professor role with the University of Minnesota, and stock options and pensions with Eli Lilly and Aetna/CVS. Dr Watkins reports payment or honoraria from ISPOR and for articles written for Value and Outcomes Spotlight, and support for attending meetings and/or travel by AMCP.
Conflict of interest statement
Drs Linnerooth, Penley, Ha, and Craven report employment with Xcenda, which provided funding for the manuscript. Drs Sauvageau and Hydery report employment Xcenda, which provided funding for the manuscript, and stock holdings with AmerisourceBergen. Dr Feeney reports support for attending meetings and/or travel provided by Highmark, Inc. Dr Thomas reports receipt of consulting fees from ActiveRADAR, board member roles with ActiveRADAR and RoundtableRx, an adjunct professor role with the University of Minnesota, and stock options and pensions with Eli Lilly and Aetna/CVS. Dr Watkins reports payment or honoraria from ISPOR and for articles written for Value and Outcomes Spotlight, and support for attending meetings and/or travel by AMCP.
Figures
Similar articles
-
Paying for Cures: How Can We Afford It? Managed Care Pharmacy Stakeholder Perceptions of Policy Options to Address Affordability of Prescription Drugs.J Manag Care Spec Pharm. 2017 Oct;23(10):1084-1090. doi: 10.18553/jmcp.2017.23.10.1084. J Manag Care Spec Pharm. 2017. PMID: 28944726 Free PMC article.
-
AMCP Partnership Forum: Enabling the Exchange of Clinical and Economic Information Pre-FDA Approval.J Manag Care Spec Pharm. 2017 Jan;23(1):105-112. doi: 10.18553/jmcp.2016.16366. Epub 2016 Dec 22. J Manag Care Spec Pharm. 2017. PMID: 28025919 Free PMC article.
-
The Current Status of Outcomes-Based Contracting for Manufacturers and Payers: An AMCP Membership Survey.J Manag Care Spec Pharm. 2018 May;24(5):410-415. doi: 10.18553/jmcp.2017.16326. Epub 2017 Dec 22. J Manag Care Spec Pharm. 2018. PMID: 29337604 Free PMC article.
-
AMCP Partnership Forum: Digital Therapeutics-What Are They and Where Do They Fit in Pharmacy and Medical Benefits?J Manag Care Spec Pharm. 2020 May;26(5):674-681. doi: 10.18553/jmcp.2020.19418. Epub 2020 Mar 16. J Manag Care Spec Pharm. 2020. PMID: 32175784 Free PMC article.
-
AMCP Partnership Forum: Optimizing Prior Authorization for Appropriate Medication Selection.J Manag Care Spec Pharm. 2020 Jan;26(1):55-62. doi: 10.18553/jmcp.2020.26.1.55. J Manag Care Spec Pharm. 2020. PMID: 31880226 Free PMC article.
Cited by
-
A primer on brand-name prescription drug contracting.J Manag Care Spec Pharm. 2024 May;30(5):507-513. doi: 10.18553/jmcp.2024.24035. Epub 2024 Apr 23. J Manag Care Spec Pharm. 2024. PMID: 38651983 Free PMC article.
-
Pathways for non-manufacturers to drive generic drug repurposing for cancer in the U.S.Front Pharmacol. 2024 Oct 9;15:1419772. doi: 10.3389/fphar.2024.1419772. eCollection 2024. Front Pharmacol. 2024. PMID: 39444616 Free PMC article.
-
A primer on formulary structures and strategies.J Manag Care Spec Pharm. 2024 Feb 3;30(2):206-210. doi: 10.18553/jmcp.2024.30.2.206. J Manag Care Spec Pharm. 2024. PMID: 38308624 Free PMC article.
-
A primer on managed care pharmacy.J Manag Care Spec Pharm. 2023 Dec;29(12):1371-1376. doi: 10.18553/jmcp.2023.29.12.1371. J Manag Care Spec Pharm. 2023. PMID: 38058142 Free PMC article.
-
Advancing the conversation: 30 years of scholarship in managed care pharmacy.J Manag Care Spec Pharm. 2025 Jul;31(7):617-626. doi: 10.18553/jmcp.2025.31.7.617. J Manag Care Spec Pharm. 2025. PMID: 40577035 Free PMC article.
References
-
- Academy of Managed Care Pharmacy. Formulary management. Published July 18, 2019. Accessed March 30, 2022. https://www.amcp.org/about/managed-care-pharmacy-101/concepts-managed-ca...
-
- US Food and Drug Administration. Drug and device manufacturer communications with payors, formulary committees, and similar entities - questions and answers. June 2018. Accessed April 4, 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents...
-
- Academy of Managed Care Pharmacy (AMCP). Bridging the HCEI divide - insights on an effective healthcare economic information (HCEI) exchange. January 19, 2022. Accessed April 1, 2022. https://www.amcp.org/Resource-Center/formulary-utilization-management/br...
MeSH terms
LinkOut - more resources
Full Text Sources