2D Nano-Sonosensitizers Facilitate Energy Transfer to Enhance Sonodynamic Therapy
- PMID: 36840977
- PMCID: PMC10175216
- DOI: 10.1002/adma.202212069
2D Nano-Sonosensitizers Facilitate Energy Transfer to Enhance Sonodynamic Therapy
Abstract
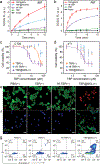
Although sonodynamic therapy (SDT) has shown promise for cancer treatment, the lack of efficient sonosensitizers (SSs) has limited the clinical application of SDT. Here, a new strategy is reported for designing efficient nano-sonosensitizers based on 2D nanoscale metal-organic layers (MOLs). Composed of Hf-oxo secondary building units (SBUs) and iridium-based linkers, the MOL is anchored with 5,10,15,20-tetra(p-benzoato)porphyrin (TBP) sensitizers on the SBUs to afford TBP@MOL. TBP@MOL shows 14.1- and 7.4-fold higher singlet oxygen (1 O2 ) generation than free TBP ligands and Hf-TBP, a 3D nanoscale metal-organic framework, respectively. The 1 O2 generation of TBP@MOL is enhanced by isolating TBP SSs on the SBUs of the MOL, which prevents aggregation-induced quenching of the excited sensitizers, and by triplet-triplet Dexter energy transfer between excited iridium-based linkers and TBP SSs, which more efficiently harnesses broad-spectrum sonoluminescence. Anchoring TBP on the MOL surface also enhances the energy transfer between the excited sensitizer and ground-state triplet oxygen to increase 1 O2 generation efficacy. In mouse models of colorectal and breast cancer, TBP@MOL demonstrates significantly higher SDT efficacy than Hf-TBP and TBP. This work uncovers a new strategy to design effective nano-sonosensitizers by facilitating energy transfer to efficiently capture broad-spectrum sonoluminescence and enhance 1 O2 generation.
Keywords: energy transfer; metal-organic layers; nanoparticles; sonodynamic therapy; sonosensitizers.
© 2023 The Authors. Advanced Materials published by Wiley-VCH GmbH.
Conflict of interest statement
Conflicts of Interest
The authors declare no competing interests.
Figures




References
-
- Brotchie A, Nature Reviews Materials 2017, 2, 17058.
-
- Choi V, Rajora MA, Zheng G, Bioconjugate Chemistry 2020, 31, 967. - PubMed
-
- Stride E, Coussios C, Nature Reviews Physics 2019, 1, 495.
-
- Son S, Kim JH, Wang X, Zhang C, Yoon SA, Shin J, Sharma A, Lee MH, Cheng L, Wu J, Kim JS, Chemical Society Reviews 2020, 49, 3244. - PubMed
-
- Qian X, Zheng Y, Chen Y, Advanced Materials 2016, 28, 8097. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

