Expression of the 2Duf protein in wild-type Bacillus subtilis spores stabilizes inner membrane proteins and increases spore resistance to wet heat and hydrogen peroxide
- PMID: 36841229
- PMCID: PMC10035073
- DOI: 10.1093/jambio/lxad040
Expression of the 2Duf protein in wild-type Bacillus subtilis spores stabilizes inner membrane proteins and increases spore resistance to wet heat and hydrogen peroxide
Abstract
Aims: This work aimed to characterize spore inner membrane (IM) properties and the mechanism of spore killing by wet heat and H2O2 with spores overexpressing the 2Duf protein, which is naturally encoded from a transposon found only in some Bacillus strains with much higher spore resistance than wild-type spores.
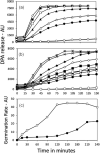
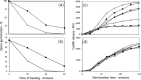
Methods and results: Killing of Bacillus subtilis spores by wet heat or hydrogen peroxide (H2O2) was slower when 2Duf was present, and Ca-dipicolinic acid release was slower than killing. Viabilities on rich plates of wet heat- or H2O2 -treated spores +/- 2Duf were lower when NaCl was added, but higher with glucose. Addition of glucose but not Casamino acids addition increased treated spores' viability on minimal medium plates. Spores with 2Duf required higher heat activation for germination, and their germination was more wet-heat resistant than that of wild-type spores, processes that involve IM proteins. IM permeability and lipid mobility were lower in spores with 2Duf, although IM phospholipid composition was similar in spores +/- 2Duf.
Conclusions: These results and previous work suggests that wet heat and H2O2 kill spores by damaging an IM enzyme or enzymes involved in oxidative phosphorylation.
Keywords: Bacillus; antimicrobials; bacterial spores; disinfection; metabolism.
© The Author(s) 2023. Published by Oxford University Press on behalf of Applied Microbiology International.
Conflict of interest statement
No conflict of interest declared.
Figures



Similar articles
-
Resistance properties and the role of the inner membrane and coat of Bacillus subtilis spores with extreme wet heat resistance.J Appl Microbiol. 2022 Mar;132(3):2157-2166. doi: 10.1111/jam.15345. Epub 2021 Nov 9. J Appl Microbiol. 2022. PMID: 34724311
-
Identification and characterization of new proteins crucial for bacterial spore resistance and germination.Front Microbiol. 2023 Apr 11;14:1161604. doi: 10.3389/fmicb.2023.1161604. eCollection 2023. Front Microbiol. 2023. PMID: 37113233 Free PMC article.
-
Treatment with oxidizing agents damages the inner membrane of spores of Bacillus subtilis and sensitizes spores to subsequent stress.J Appl Microbiol. 2004;97(4):838-52. doi: 10.1111/j.1365-2672.2004.02370.x. J Appl Microbiol. 2004. PMID: 15357734
-
Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals.J Appl Microbiol. 2006 Sep;101(3):514-25. doi: 10.1111/j.1365-2672.2005.02736.x. J Appl Microbiol. 2006. PMID: 16907802 Review.
-
Germination of spores of Bacillus species: what we know and do not know.J Bacteriol. 2014 Apr;196(7):1297-305. doi: 10.1128/JB.01455-13. Epub 2014 Jan 31. J Bacteriol. 2014. PMID: 24488313 Free PMC article. Review.
Cited by
-
Modeling heterogeneity, commitment, and memory of bacterial spore germination.mBio. 2025 May 14;16(5):e0059625. doi: 10.1128/mbio.00596-25. Epub 2025 Apr 2. mBio. 2025. PMID: 40172195 Free PMC article.
-
Pathways for accelerated bacterial spore killing with ohmic heating.NPJ Sci Food. 2025 Aug 7;9(1):167. doi: 10.1038/s41538-025-00537-1. NPJ Sci Food. 2025. PMID: 40775241 Free PMC article.
-
The megaplasmid pCER270 of Bacillus cereus emetic strain affects the timing of the sporulation process, spore resistance properties, and germination.Appl Environ Microbiol. 2024 Sep 18;90(9):e0102924. doi: 10.1128/aem.01029-24. Epub 2024 Aug 19. Appl Environ Microbiol. 2024. PMID: 39158315 Free PMC article.
-
Germination and Heat Resistance of Parageobacillus and Geobacillus spp. Spores.Foods. 2025 Jun 11;14(12):2061. doi: 10.3390/foods14122061. Foods. 2025. PMID: 40565670 Free PMC article.
-
New Thoughts on an Old Topic: Secrets of Bacterial Spore Resistance Slowly Being Revealed.Microbiol Mol Biol Rev. 2023 Jun 28;87(2):e0008022. doi: 10.1128/mmbr.00080-22. Epub 2023 Mar 16. Microbiol Mol Biol Rev. 2023. PMID: 36927044 Free PMC article. Review.

