Toxicity and assimilation of cellulosic copper nanoparticles require α-arrestins in S. cerevisiae
- PMID: 36841230
- PMCID: PMC10022662
- DOI: 10.1093/mtomcs/mfad011
Toxicity and assimilation of cellulosic copper nanoparticles require α-arrestins in S. cerevisiae
Abstract
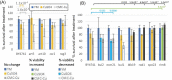
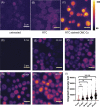
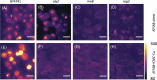
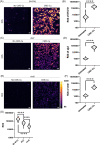
The increased use of antimicrobial compounds such as copper into nanoparticles changes how living cells interact with these novel materials. The increased use of antimicrobial nanomaterials combats infectious disease and food spoilage. Fungal infections are particularly difficult to treat because of the few druggable targets, and Saccharomyces cerevisiae provides an insightful model organism to test these new materials. However, because of the novel characteristics of these materials, it is unclear how these materials interact with living cells and if resistance to copper-based nanomaterials could occur. Copper nanoparticles built on carboxymethylcellulose microfibril strands with copper (CMC-Cu) are a promising nanomaterial when imported into yeast cells and induce cell death. The α-arrestins are cargo adaptors that select which molecules are imported into eukaryotic cells. We screened α-arrestins mutants and identified Aly2, Rim8, and Rog3 α-arrestins, which are necessary for the internalization of CMC-Cu nanoparticles. Internal reactive oxygen species in these mutants were lower and corresponded to the increased viability in the presence of CMC-Cu. Using lattice light-sheet microscopy on live cells, we determined that CMC-Cu were imported into yeast within 30 min of exposure. Initially, the cytoplasmic pH decreased but returned to basal level 90 min later. However, there was heterogeneity in response to CMC-Cu exposure, which could be due to the heterogeneity of the particles or differences in the metabolic states within the population. When yeast were exposed to sublethal concentrations of CMC-Cu no resistance occurred. Internalization of CMC-Cu increases the potency of these antimicrobial nanomaterials and is likely key to preventing fungi from evolving resistance.
Keywords: S. cerevisiae; copper nanoparticles; endocytosis; lattice light-sheet microscopy; reactive oxygen species; α-arrestins.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Shi Z., Zang S., Jiang F., Huang L., Lu D., Ma Y., Yang G., In Situ nano-assembly of bacterial cellulose-polyaniline composites, RSC Adv., 2012, 2 (3), 1040–1046. 10.1039/c1ra00719j - DOI
-
- Zhong T., Oporto G. S., Armstrong J., Jaczynski J., Tesfai A. T., Antimicrobial properties of the hybrid copper nanoparticles-carboxymethyl cellulose, Wood Fiber Sci, 2013, 45, 215–222.
-
- Cioffi N., Ditaranto N., Torsi L., Sabbatini L., Approaches to synthesis and characterization of spherical and anisotropic copper nanomaterials, Nanotechnol. Life Sci., 2010, 1, 3–69.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

