The effect of BCG vaccination on infection and antibody levels against SARS-CoV-2-The results of ProBCG: a multicenter randomized clinical trial in Brazil
- PMID: 36841502
- PMCID: PMC9972589
- DOI: 10.1016/j.ijid.2023.02.014
The effect of BCG vaccination on infection and antibody levels against SARS-CoV-2-The results of ProBCG: a multicenter randomized clinical trial in Brazil
Abstract
Objectives: Evatuate if Bacillus Calmette-Guérin (BCG) vaccine could be used as a tool against SARS-CoV-2 based on the concept of trained immunity.
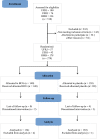
Methods: A multicenter, double-blinded, randomized clinical trial recruited health care workers (HCWs) in Brazil. The incidence rates of COVID-19, clinical manifestations, absenteeism, and adverse events among HCWs receiving BCG vaccine (Moreau or Moscow strains) or placebo were compared. BCG vaccine-mediated immune response before and after implementing specific vaccines for COVID-19 (CoronaVac or COVISHIELD) was analyzed. Cox proportional hazard and linear mixed effect modeling were used.
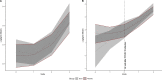
Results: A total of 264 volunteers were included for analysis (BCG = 134 and placebo = 130). The placebo group presented a COVID-19 cumulative incidence of 0.75% vs 0.52% of BCG. The Moreau strain also presented a higher incidence rate (1.60% × 0.22%). BCG did not show a protective hazard ratio against COVID-19. In addition, the log (immunoglobulin G) level against SARS-CoV-2 presented a higher increase in the BCG group, whether or not participants had COVID-19, but also without statistical significance.
Conclusion: Our results suggest that BCG has a tendency of protection against SARS-CoV-2 and higher immunoglobulin G levels than placebo. The clinical trial was registered at https://clinicaltrials.gov/ (NCT04659941).
Keywords: BCG; COVID-19; Vaccine.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration Of Competing Interest The authors have no competing interests to declare.
Figures

References
-
- World Health Organization. Director General's Opening Remarks at the Media Briefing on COVID-19, https://covid19.who.int/2020 [accessed 09 September 2022].
-
- Miller A, Raendelar MJ, Fasciglione K, Roumenova V, Li Y, Otazu GH. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. medRxiv. 28 March 2020. https://www.medrxiv.org/content/10.1101/2020.03.24.20042937v1 [accessed 10 August 2022]. - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

