NRP1 promotes prostate cancer progression via modulating EGFR-dependent AKT pathway activation
- PMID: 36841806
- PMCID: PMC9958327
- DOI: 10.1038/s41419-023-05696-1
NRP1 promotes prostate cancer progression via modulating EGFR-dependent AKT pathway activation
Abstract
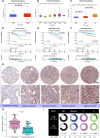
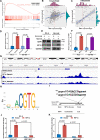
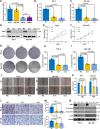
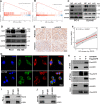
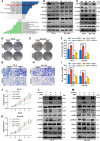
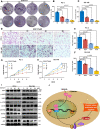
Prostate cancer (PCa) is the most common malignant tumor with a high global incidence in males. The mechanism underlying PCa progression is still not clear. This study observed that NRP1 was highly expressed in PCa and associated with poor prognosis in PCa patients. Functionally, NRP1 depletion attenuated the proliferation and migration ability of PCa cells in vitro and in vivo, while NRP1 overexpression promoted PCa cell proliferation and migration. Moreover, it was observed that NRP1 depletion induced G1 phase arrest in PCa cells. Mechanistically, HIF1α is bound to the specific promoter region of NRP1, thereby regulating its transcriptional activation. Subsequently, NRP1 interacted with EGFR, leading to EGFR phosphorylation. This study also provided evidence that the b1/b2 domain of NRP1 was responsible for the interaction with the extracellular domain of EGFR. Moreover, EGFR mediated NRP1-induced activation of the AKT signaling pathway, which promoted the malignant progression of PCa. In addition, the administration of NRP1 inhibitor EG01377 significantly inactivated the EGFR/AKT signaling axis, thereby suppressing PCa progression. In conclusion, the findings from this study highlighted the molecular mechanism underlying NRP1 expression in PCa and provide a potential predictor and therapeutic target for clinical prognosis and treatment of PCa.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
CMTM5 inhibits the development of prostate cancer via the EGFR/PI3K/AKT signaling pathway.Mol Med Rep. 2022 Jan;25(1):17. doi: 10.3892/mmr.2021.12533. Epub 2021 Nov 18. Mol Med Rep. 2022. PMID: 34791506 Free PMC article.
-
LncRNA RHPN1-AS1 inhibition induces autophagy and apoptosis in prostate cancer cells via the miR-7-5p/EGFR/PI3K/AKT/mTOR signaling pathway.Environ Toxicol. 2022 Dec;37(12):3013-3027. doi: 10.1002/tox.23656. Epub 2022 Sep 20. Environ Toxicol. 2022. PMID: 36125241
-
Vascular endothelial growth factor regulates myeloid cell leukemia-1 expression through neuropilin-1-dependent activation of c-MET signaling in human prostate cancer cells.Mol Cancer. 2010 Jan 19;9:9. doi: 10.1186/1476-4598-9-9. Mol Cancer. 2010. PMID: 20085644 Free PMC article.
-
The regulation of Neuropilin 1 expression by miR-338-3p promotes non-small cell lung cancer via changes in EGFR signaling.Mol Carcinog. 2019 Jun;58(6):1019-1032. doi: 10.1002/mc.22990. Epub 2019 Feb 27. Mol Carcinog. 2019. PMID: 30811684 Free PMC article.
-
The PI3K/AKT pathway in the pathogenesis of prostate cancer.Front Biosci (Landmark Ed). 2016 Jun 1;21(5):1084-91. doi: 10.2741/4443. Front Biosci (Landmark Ed). 2016. PMID: 27100493 Review.
Cited by
-
Pan-Cancer Analysis of the Prognostic and Immunological Role of SEMA7A.Int J Gen Med. 2024 Dec 25;17:6443-6461. doi: 10.2147/IJGM.S499872. eCollection 2024. Int J Gen Med. 2024. PMID: 39735166 Free PMC article.
-
An early-onset specific polygenic risk score optimizes age-based risk estimate and stratification of prostate cancer: population-based cohort study.J Transl Med. 2024 Apr 17;22(1):366. doi: 10.1186/s12967-024-05190-y. J Transl Med. 2024. PMID: 38632662 Free PMC article.
-
Integrated analysis of disulfidoptosis-related genes identifies NRP1 as a novel biomarker promoting proliferation of gastric cancer via glutamine mediated energy metabolism.Discov Oncol. 2024 Aug 7;15(1):337. doi: 10.1007/s12672-024-01217-4. Discov Oncol. 2024. PMID: 39110136 Free PMC article.
-
β-Asarone Inhibits Carboplatin Resistance in Retinoblastoma Cells Through the UCA1/miR-206/NRP1 Axis.Biochem Genet. 2024 Dec 24. doi: 10.1007/s10528-024-10985-1. Online ahead of print. Biochem Genet. 2024. PMID: 39718722
-
A disulfidptosis-related classification and risk signature identifies immunotherapy biomarkers and predicts prognosis in gastric cancer: An observational study.Medicine (Baltimore). 2024 May 31;103(22):e38398. doi: 10.1097/MD.0000000000038398. Medicine (Baltimore). 2024. PMID: 39259065 Free PMC article.
References
-
- Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. Part II-2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2021;79:263–82. doi: 10.1016/j.eururo.2020.09.046. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

