Biomineralization-inspired mineralized hydrogel promotes the repair and regeneration of dentin/bone hard tissue
- PMID: 36841873
- PMCID: PMC9968336
- DOI: 10.1038/s41536-023-00286-3
Biomineralization-inspired mineralized hydrogel promotes the repair and regeneration of dentin/bone hard tissue
Abstract
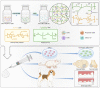
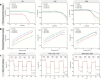
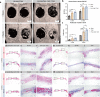
Maxillofacial hard tissue defects caused by trauma or infection often affect craniofacial function. Taking the natural hard tissue structure as a template, constructing an engineered tissue repair module is an important scheme to realize the functional regeneration and repair of maxillofacial hard tissue. Here, inspired by the biomineralization process, we constructed a composite mineral matrix hydrogel PAA-CMC-TDM containing amorphous calcium phosphates (ACPs), polyacrylic acid (PAA), carboxymethyl chitosan (CMC) and dentin matrix (TDM). The dynamic network composed of Ca2+·COO- coordination and ACPs made the hydrogel loaded with TDM, and exhibited self-repairing ability and injectability. The mechanical properties of PAA-CMC-TDM can be regulated, but the functional activity of TDM remains unaffected. Cytological studies and animal models of hard tissue defects show that the hydrogel can promote the odontogenesis or osteogenic differentiation of mesenchymal stem cells, adapt to irregular hard tissue defects, and promote in situ regeneration of defective tooth and bone tissues. In summary, this paper shows that the injectable TDM hydrogel based on biomimetic mineralization theory can induce hard tissue formation and promote dentin/bone regeneration.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
Facile Synthesis of In Situ Formable Alginate Composite Hydrogels with Ca2+-Induced Healing Ability.Tissue Eng Part A. 2021 Oct;27(19-20):1225-1238. doi: 10.1089/ten.TEA.2020.0282. Epub 2021 Feb 15. Tissue Eng Part A. 2021. PMID: 33323027
-
Chitosan-Based Biomimetically Mineralized Composite Materials in Human Hard Tissue Repair.Molecules. 2020 Oct 19;25(20):4785. doi: 10.3390/molecules25204785. Molecules. 2020. PMID: 33086470 Free PMC article. Review.
-
Demineralized Dentin Matrix for Dental and Alveolar Bone Tissues Regeneration: An Innovative Scope Review.Tissue Eng Regen Med. 2022 Aug;19(4):687-701. doi: 10.1007/s13770-022-00438-4. Epub 2022 Apr 16. Tissue Eng Regen Med. 2022. PMID: 35429315 Free PMC article. Review.
-
Cryopreserved dentin matrix as a scaffold material for dentin-pulp tissue regeneration.Biomaterials. 2014 Jun;35(18):4929-39. doi: 10.1016/j.biomaterials.2014.03.016. Epub 2014 Mar 27. Biomaterials. 2014. PMID: 24680189
-
Photocrosslinkable gelatin-treated dentin matrix hydrogel as a novel pulp capping agent for dentin regeneration: I. synthesis, characterizations and grafting optimization.BMC Oral Health. 2023 Aug 4;23(1):536. doi: 10.1186/s12903-023-03236-z. BMC Oral Health. 2023. PMID: 37542230 Free PMC article.
Cited by
-
A wearable electrostimulation-augmented ionic-gel photothermal patch doped with MXene for skin tumor treatment.Nat Commun. 2024 Jan 26;15(1):762. doi: 10.1038/s41467-024-45070-z. Nat Commun. 2024. PMID: 38278810 Free PMC article.
-
Augmenting osteoporotic osseointegration through a temporal release nanocoating-based reversing dysregulated osteogenic microenvironment.J Orthop Translat. 2025 Apr 5;51:360-378. doi: 10.1016/j.jot.2025.01.009. eCollection 2025 Mar. J Orthop Translat. 2025. PMID: 40584018 Free PMC article.
-
Biomineral-Based Composite Materials in Regenerative Medicine.Int J Mol Sci. 2024 Jun 2;25(11):6147. doi: 10.3390/ijms25116147. Int J Mol Sci. 2024. PMID: 38892335 Free PMC article. Review.
-
Anisotropic Liesegang pattern for the nonlinear elastic biomineral-hydrogel complex.Sci Adv. 2024 Apr 26;10(17):eadl3075. doi: 10.1126/sciadv.adl3075. Epub 2024 Apr 26. Sci Adv. 2024. PMID: 38669324 Free PMC article.
-
Mechanobiology of Dental Pulp Cells.Cells. 2024 Feb 21;13(5):375. doi: 10.3390/cells13050375. Cells. 2024. PMID: 38474339 Free PMC article. Review.
References
-
- Liu J, Zhang SS, Zheng SG, Xu T, Si Y. Oral health status and oral health care model in China. Chin. J. Dent. Res. 2016;19:207–215. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

