Intra-pituitary follicle-stimulating hormone signaling regulates hepatic lipid metabolism in mice
- PMID: 36841874
- PMCID: PMC9968338
- DOI: 10.1038/s41467-023-36681-z
Intra-pituitary follicle-stimulating hormone signaling regulates hepatic lipid metabolism in mice
Abstract
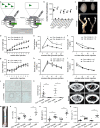
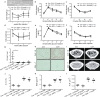
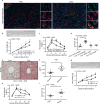
Inter-organ communication is a major hallmark of health and is often orchestrated by hormones released by the anterior pituitary gland. Pituitary gonadotropes secrete follicle-stimulating hormone (FSH) and luteinizing hormone (LH) to regulate gonadal function and control fertility. Whether FSH and LH also act on organs other than the gonads is debated. Here, we find that gonadotrope depletion in adult female mice triggers profound hypogonadism, obesity, glucose intolerance, fatty liver, and bone loss. The absence of sex steroids precipitates these phenotypes, with the notable exception of fatty liver, which results from ovary-independent actions of FSH. We uncover paracrine FSH action on pituitary corticotropes as a mechanism to restrain the production of corticosterone and prevent hepatic steatosis. Our data demonstrate that functional communication of two distinct hormone-secreting cell populations in the pituitary regulates hepatic lipid metabolism.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

