Membrane phospholipids control gating of the mechanosensitive potassium leak channel TREK1
- PMID: 36841877
- PMCID: PMC9968290
- DOI: 10.1038/s41467-023-36765-w
Membrane phospholipids control gating of the mechanosensitive potassium leak channel TREK1
Abstract
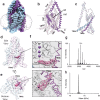
Tandem pore domain (K2P) potassium channels modulate resting membrane potentials and shape cellular excitability. For the mechanosensitive subfamily of K2Ps, the composition of phospholipids within the bilayer strongly influences channel activity. To examine the molecular details of K2P lipid modulation, we solved cryo-EM structures of the TREK1 K2P channel bound to either the anionic lipid phosphatidic acid (PA) or the zwitterionic lipid phosphatidylethanolamine (PE). At the extracellular face of TREK1, a PA lipid inserts its hydrocarbon tail into a pocket behind the selectivity filter, causing a structural rearrangement that recapitulates mutations and pharmacology known to activate TREK1. At the cytoplasmic face, PA and PE lipids compete to modulate the conformation of the TREK1 TM4 gating helix. Our findings demonstrate two distinct pathways by which anionic lipids enhance TREK1 activity and provide a framework for a model that integrates lipid gating with the effects of other mechanosensitive K2P modulators.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



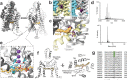

Similar articles
-
Structural Insights into the Mechanisms and Pharmacology of K2P Potassium Channels.J Mol Biol. 2021 Aug 20;433(17):166995. doi: 10.1016/j.jmb.2021.166995. Epub 2021 Apr 20. J Mol Biol. 2021. PMID: 33887333 Free PMC article. Review.
-
How ion channels sense mechanical force: insights from mechanosensitive K2P channels TRAAK, TREK1, and TREK2.Ann N Y Acad Sci. 2015 Sep;1352:20-32. doi: 10.1111/nyas.12874. Epub 2015 Aug 31. Ann N Y Acad Sci. 2015. PMID: 26332952 Review.
-
Effect of two activators on the gating of a K2P channel.Biophys J. 2024 Oct 1;123(19):3408-3420. doi: 10.1016/j.bpj.2024.08.006. Epub 2024 Aug 19. Biophys J. 2024. PMID: 39161093 Free PMC article.
-
The potassium channels TASK2 and TREK1 regulate functional differentiation of murine skeletal muscle cells.Am J Physiol Cell Physiol. 2016 Oct 1;311(4):C583-C595. doi: 10.1152/ajpcell.00363.2015. Epub 2016 Aug 3. Am J Physiol Cell Physiol. 2016. PMID: 27488672
-
Bilayer-Mediated Structural Transitions Control Mechanosensitivity of the TREK-2 K2P Channel.Structure. 2017 May 2;25(5):708-718.e2. doi: 10.1016/j.str.2017.03.006. Epub 2017 Apr 6. Structure. 2017. PMID: 28392258 Free PMC article.
Cited by
-
C-type inactivation and proton modulation mechanisms of the TASK3 channel.Proc Natl Acad Sci U S A. 2024 Apr 23;121(17):e2320345121. doi: 10.1073/pnas.2320345121. Epub 2024 Apr 17. Proc Natl Acad Sci U S A. 2024. PMID: 38630723 Free PMC article.
-
Cannabinoid inhibition of mechanosensitive K+ channels.bioRxiv [Preprint]. 2024 Dec 10:2024.12.09.627564. doi: 10.1101/2024.12.09.627564. bioRxiv. 2024. PMID: 39713384 Free PMC article. Preprint.
-
Multiple-Basin Go̅-Martini for Investigating Conformational Transitions and Environmental Interactions of Proteins.J Chem Theory Comput. 2025 May 27;21(10):5304-5321. doi: 10.1021/acs.jctc.5c00256. Epub 2025 May 13. J Chem Theory Comput. 2025. PMID: 40359486 Free PMC article.
-
Atomistic mechanism of noncanonical voltage gating in K2P channels.Sci Adv. 2025 Aug 8;11(32):eadx1680. doi: 10.1126/sciadv.adx1680. Epub 2025 Aug 6. Sci Adv. 2025. PMID: 40768588 Free PMC article.
-
Structure of human PIEZO1 and its slow-inactivating channelopathy mutants.Elife. 2025 Jul 16;13:RP101923. doi: 10.7554/eLife.101923. Elife. 2025. PMID: 40668110 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

