mRNA targeting eliminates the need for the signal recognition particle during membrane protein insertion in bacteria
- PMID: 36842086
- PMCID: PMC10066597
- DOI: 10.1016/j.celrep.2023.112140
mRNA targeting eliminates the need for the signal recognition particle during membrane protein insertion in bacteria
Abstract
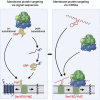
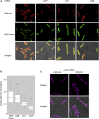
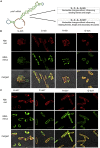
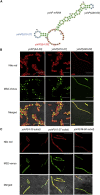
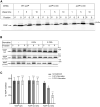
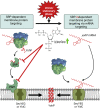
Signal-sequence-dependent protein targeting is essential for the spatiotemporal organization of eukaryotic and prokaryotic cells and is facilitated by dedicated protein targeting factors such as the signal recognition particle (SRP). However, targeting signals are not exclusively contained within proteins but can also be present within mRNAs. By in vivo and in vitro assays, we show that mRNA targeting is controlled by the nucleotide content and by secondary structures within mRNAs. mRNA binding to bacterial membranes occurs independently of soluble targeting factors but is dependent on the SecYEG translocon and YidC. Importantly, membrane insertion of proteins translated from membrane-bound mRNAs occurs independently of the SRP pathway, while the latter is strictly required for proteins translated from cytosolic mRNAs. In summary, our data indicate that mRNA targeting acts in parallel to the canonical SRP-dependent protein targeting and serves as an alternative strategy for safeguarding membrane protein insertion when the SRP pathway is compromised.
Keywords: (p)ppGpp; CP: Microbiology; FtsY; SecYEG translocon; YidC; alarmones; mRNA targeting; signal recognition particle; small membrane proteins; stringent response; translation.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


Similar articles
-
Predominant membrane localization is an essential feature of the bacterial signal recognition particle receptor.BMC Biol. 2009 Nov 13;7:76. doi: 10.1186/1741-7007-7-76. BMC Biol. 2009. PMID: 19912622 Free PMC article.
-
Posttranslational insertion of small membrane proteins by the bacterial signal recognition particle.PLoS Biol. 2020 Sep 30;18(9):e3000874. doi: 10.1371/journal.pbio.3000874. eCollection 2020 Sep. PLoS Biol. 2020. PMID: 32997663 Free PMC article.
-
Promiscuous targeting of polytopic membrane proteins to SecYEG or YidC by the Escherichia coli signal recognition particle.Mol Biol Cell. 2012 Feb;23(3):464-79. doi: 10.1091/mbc.E11-07-0590. Epub 2011 Dec 7. Mol Biol Cell. 2012. PMID: 22160593 Free PMC article.
-
Targeting and Insertion of Membrane Proteins.EcoSal Plus. 2017 Mar;7(2):10.1128/ecosalplus.ESP-0012-2016. doi: 10.1128/ecosalplus.ESP-0012-2016. EcoSal Plus. 2017. PMID: 28276312 Free PMC article. Review.
-
Co-translational protein targeting in bacteria.FEMS Microbiol Lett. 2018 Jun 1;365(11). doi: 10.1093/femsle/fny095. FEMS Microbiol Lett. 2018. PMID: 29790984 Review.
Cited by
-
Control of a chemical chaperone by a universally conserved ATPase.iScience. 2024 Jun 8;27(7):110215. doi: 10.1016/j.isci.2024.110215. eCollection 2024 Jul 19. iScience. 2024. PMID: 38993675 Free PMC article.
-
Essential factors, advanced strategies, challenges, and approaches involved for efficient expression of recombinant proteins in Escherichia coli.Arch Microbiol. 2024 Mar 12;206(4):152. doi: 10.1007/s00203-024-03871-2. Arch Microbiol. 2024. PMID: 38472371 Review.
-
snoRNA-facilitated protein secretion revealed by transcriptome-wide snoRNA target identification.Cell. 2025 Jan 23;188(2):465-483.e22. doi: 10.1016/j.cell.2024.10.046. Epub 2024 Nov 22. Cell. 2025. PMID: 39579764
-
Highly Multiplexed Spatial Transcriptomics in Bacteria.bioRxiv [Preprint]. 2024 Jun 27:2024.06.27.601034. doi: 10.1101/2024.06.27.601034. bioRxiv. 2024. Update in: Science. 2025 Jan 24;387(6732):eadr0932. doi: 10.1126/science.adr0932. PMID: 38979245 Free PMC article. Updated. Preprint.
-
YidC from Escherichia coli Forms an Ion-Conducting Pore upon Activation by Ribosomes.Biomolecules. 2023 Dec 11;13(12):1774. doi: 10.3390/biom13121774. Biomolecules. 2023. PMID: 38136645 Free PMC article.
References
-
- Müller M., Koch H.G., Beck K., Schäfer U. Protein traffic in bacteria: multiple routes from the ribosome to and across the membrane. Prog. Nucleic Acid Res. Mol. Biol. 2001;66:107–157. - PubMed
-
- Zimmermann R., Eyrisch S., Ahmad M., Helms V. Protein translocation across the ER membrane. Biochim. Biophys. Acta. 2011;1808:912–924. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

