mRNA targeting eliminates the need for the signal recognition particle during membrane protein insertion in bacteria
- PMID: 36842086
- PMCID: PMC10066597
- DOI: 10.1016/j.celrep.2023.112140
mRNA targeting eliminates the need for the signal recognition particle during membrane protein insertion in bacteria
Abstract
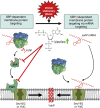
Signal-sequence-dependent protein targeting is essential for the spatiotemporal organization of eukaryotic and prokaryotic cells and is facilitated by dedicated protein targeting factors such as the signal recognition particle (SRP). However, targeting signals are not exclusively contained within proteins but can also be present within mRNAs. By in vivo and in vitro assays, we show that mRNA targeting is controlled by the nucleotide content and by secondary structures within mRNAs. mRNA binding to bacterial membranes occurs independently of soluble targeting factors but is dependent on the SecYEG translocon and YidC. Importantly, membrane insertion of proteins translated from membrane-bound mRNAs occurs independently of the SRP pathway, while the latter is strictly required for proteins translated from cytosolic mRNAs. In summary, our data indicate that mRNA targeting acts in parallel to the canonical SRP-dependent protein targeting and serves as an alternative strategy for safeguarding membrane protein insertion when the SRP pathway is compromised.
Keywords: (p)ppGpp; CP: Microbiology; FtsY; SecYEG translocon; YidC; alarmones; mRNA targeting; signal recognition particle; small membrane proteins; stringent response; translation.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- Müller M., Koch H.G., Beck K., Schäfer U. Protein traffic in bacteria: multiple routes from the ribosome to and across the membrane. Prog. Nucleic Acid Res. Mol. Biol. 2001;66:107–157. - PubMed
-
- Zimmermann R., Eyrisch S., Ahmad M., Helms V. Protein translocation across the ER membrane. Biochim. Biophys. Acta. 2011;1808:912–924. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

