Ribosomal DNA replication time coordinates completion of genome replication and anaphase in yeast
- PMID: 36842087
- PMCID: PMC10142053
- DOI: 10.1016/j.celrep.2023.112161
Ribosomal DNA replication time coordinates completion of genome replication and anaphase in yeast
Abstract
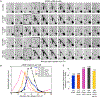
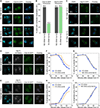
Timely completion of genome replication is a prerequisite for mitosis, genome integrity, and cell survival. A challenge to this timely completion comes from the need to replicate the hundreds of untranscribed copies of rDNA that organisms maintain in addition to the copies required for ribosome biogenesis. Replication of these rDNA arrays is relegated to late S phase despite their large size, repetitive nature, and essentiality. Here, we show that, in Saccharomyces cerevisiae, reducing the number of rDNA repeats leads to early rDNA replication, which results in delaying replication elsewhere in the genome. Moreover, cells with early-replicating rDNA arrays and delayed genome-wide replication aberrantly release the mitotic phosphatase Cdc14 from the nucleolus and enter anaphase prematurely. We propose that rDNA copy number determines the replication time of the rDNA locus and that the release of Cdc14 upon completion of rDNA replication is a signal for cell cycle progression.
Keywords: CP: Molecular biology; Cdc14; Fob1; anaphase; cell cycle; copy number variation; nucleolus; origin of replication; rDNA; replication timing; ribosomal DNA.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests C.Q. is a Cell Reports Advisory Board member, Ecology and Evolution.
Figures




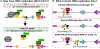
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

