Immune-related adverse events as potential surrogates of immune checkpoint inhibitors' efficacy: a systematic review and meta-analysis of randomized studies
- PMID: 36842300
- PMCID: PMC9984799
- DOI: 10.1016/j.esmoop.2023.100787
Immune-related adverse events as potential surrogates of immune checkpoint inhibitors' efficacy: a systematic review and meta-analysis of randomized studies
Abstract
Background: Immune-related adverse events (irAEs) are frequently reported during immune checkpoint inhibitor (ICI) therapy and are associated with long-term outcomes. It is unknown if the irAE occurrence is a valid surrogate of ICIs' efficacy.
Methods: We identified articles reporting the results of randomized trials of experimental ICI therapy in solid tumors with a systematic search. The control arms could be placebo, cytotoxic/targeted therapy, or ICI therapy. We extracted the hazard ratios for overall survival (OS) with the number of OS events per arm and the number and percentages of overall and specific irAEs of grade 1-2 and grade 3-4 per arm. We estimated the treatment effect on the potential surrogate outcome with the odds ratio of the irAE rate between the experimental and the control arm. The statistical analysis consisted of weighted linear regression on a logarithmic scale between treatment effects on irAE rate and treatment effects on OS.
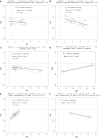
Results: Sixty-two randomized trials were included for a total of 79 contrasts and 42 247 patients. The analyses found no significant association between the treatment effects for overall grade 1-2 or grade 3-4 irAE rates or specific (skin, gastrointestinal, endocrine) irAE rates. In the non-small-cell lung cancer (NSCLC) trial subset, we observed a negative association between treatment effects on overall grade 1-2 irAEs and treatment effects on OS in studies with patients selected for programmed death-ligand 1 expression (R2 = 0.55; 95% confidence interval 0.20-0.95; R = -0.69). In the melanoma trial subset, a negative association was shown between treatment effects on gastrointestinal grade 3-4 irAEs and treatment effects on OS in trials without an ICI-based control arm (R2 = 0.77; 95% confidence interval 0.24-0.99; R = -0.89).
Conclusions: We found low-strength correlations between the ICI therapy effects on overall or specific irAE rates and the treatment effects on OS in several cancer types.
Keywords: immune checkpoint inhibitors; immune-related adverse events; surrogate endpoints; trial-based meta-analysis.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures




References
-
- Koon H., Atkins M. Autoimmunity and immunotherapy for cancer. N Engl J Med. 2006;354(7):758–760. - PubMed
-
- Zimmer L., Goldinger S.M., Hofmann L., et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:210–225. - PubMed
-
- Hofmann L., Forschner A., Loquai C., et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190–209. - PubMed
-
- Boutros C., Tarhini A., Routier E., et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13(8):473–486. - PubMed
-
- Haanen J., Carbonnel F., Robert C., et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(suppl 4):iv264–iv266. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

