Precision oncology for BRAF-mutant cancers with BRAF and MEK inhibitors: from melanoma to tissue-agnostic therapy
- PMID: 36842301
- PMCID: PMC9984800
- DOI: 10.1016/j.esmoop.2023.100788
Precision oncology for BRAF-mutant cancers with BRAF and MEK inhibitors: from melanoma to tissue-agnostic therapy
Abstract
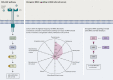
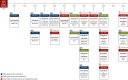
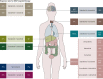
BRAF activation occurs as part of the mitogen-activated protein kinase (MAPK) cellular signaling pathway which leads to increased cellular proliferation and survival. Mutations in BRAF can result in unbridled activation of downstream kinases with subsequent uncontrolled cellular growth that formulate the basis for oncogenesis in multiple tumor types. Targeting BRAF by selective inhibitors has been one of the early successes in precision oncology. Agents have been explored either as monotherapy or in combination with MEK inhibition in BRAF V600-mutant pan-cancers and with EGFR inhibition in colorectal cancer. Spectrum of BRAF inhibition has evolved from being melanoma-specific to being a pan-cancer target. In this article, we review BRAF and MEK inhibitor drug development journey from tissue-specific melanoma, non-small-cell lung cancer, and anaplastic thyroid cancer to tissue-agnostic approvals.
Keywords: BRAF; MEK; cancer; precision oncology; targeted therapy.
Copyright © 2023 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Wellbrock C., Karasarides M., Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875–885. - PubMed
-
- Pearson G., Robinson F., Beers Gibson T., et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. - PubMed
-
- Subbiah V., Baik C., Kirkwood J.M. Clinical development of BRAF plus MEK inhibitor combinations. Trends Cancer. 2020;6:797–810. - PubMed
-
- Davies H., Bignell G.R., Cox C., et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

