Skin γδ T cell inflammatory responses are hardwired in the thymus by oxysterol sensing via GPR183 and calibrated by dietary cholesterol
- PMID: 36842431
- PMCID: PMC10306310
- DOI: 10.1016/j.immuni.2023.01.025
Skin γδ T cell inflammatory responses are hardwired in the thymus by oxysterol sensing via GPR183 and calibrated by dietary cholesterol
Abstract
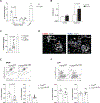
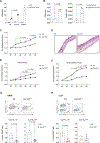
Dietary components and metabolites have a profound impact on immunity and inflammation. Here, we investigated how sensing of cholesterol metabolite oxysterols by γδ T cells impacts their tissue residency and function. We show that dermal IL-17-producing γδ T (Tγδ17) cells essential for skin-barrier homeostasis require oxysterols sensing through G protein receptor 183 (GPR183) for their development and inflammatory responses. Single-cell transcriptomics and murine reporter strains revealed that GPR183 on developing γδ thymocytes is needed for their maturation by sensing medullary thymic epithelial-cell-derived oxysterols. In the skin, basal keratinocytes expressing the oxysterol enzyme cholesterol 25-hydroxylase (CH25H) maintain dermal Tγδ17 cells. Diet-driven increases in oxysterols exacerbate Tγδ17-cell-mediated psoriatic inflammation, dependent on GPR183 on γδ T cells. Hence, cholesterol-derived oxysterols control spatially distinct but biologically linked processes of thymic education and peripheral function of dermal T cells, implicating diet as a focal parameter of dermal Tγδ17 cells.
Keywords: GPR183; IL-17; cholesterol; diet; gamma delta T cells; mTEC; oxysterols; psoriasis; skin.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

