Age-related changes in basal forebrain afferent activation in response to food paired stimuli
- PMID: 36842481
- PMCID: PMC10155118
- DOI: 10.1016/j.neulet.2023.137155
Age-related changes in basal forebrain afferent activation in response to food paired stimuli
Abstract
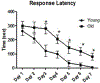
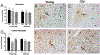
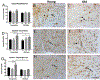
The basal forebrain contains a phenotypically-diverse assembly of neurons, including those using acetylcholine as their neurotransmitter. This basal forebrain cholinergic system projects to the entire neocortical mantle as well as subcortical limbic structures that include the hippocampus and amygdala. Basal forebrain pathology, including cholinergic dysfunction, is thought to underlie the cognitive impairments associated with several age-related neurodegenerative conditions, including Alzheimer's disease. Basal forebrain dysfunction may stem, in part, from a failure of normal afferent regulation of cholinergic and other neurons in this area. However, little is understood regarding how aging, alone, affects the functional regulation of basal forebrain afferents in the context of motivated behavior. Here, we used neuronal tract-tracing combined with motivationally salient stimuli in an aged rodent model to examine how aging alters activity in basal forebrain inputs arising from several cortical, limbic and brainstem structures. Young rats showed greater stimulus-associated activation of basal forebrain inputs arising from prelimbic cortex, nucleus accumbens and the ventral tegmental area compared with aged rats. Aged rats also showed increased latency to respond to palatable food presentation compared to young animals. Changes in activation of intrinsic basal forebrain cell populations or afferents were also observed as a function of age or experimental condition. These data further demonstrate that age-related changes in basal forebrain activation and related behavioral and cognitive functions reflect a failure of afferent regulation of this important brain region.
Keywords: Aging; Basal forebrain; Cholinergic; Feeding; Motivated behavior; Tract-tracing.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Cortical cholinergic inputs mediating arousal, attentional processing and dreaming: differential afferent regulation of the basal forebrain by telencephalic and brainstem afferents.Neuroscience. 2000;95(4):933-52. doi: 10.1016/s0306-4522(99)00487-x. Neuroscience. 2000. PMID: 10682701 Review.
-
Neuronal amyloid-β accumulation within cholinergic basal forebrain in ageing and Alzheimer's disease.Brain. 2015 Jun;138(Pt 6):1722-37. doi: 10.1093/brain/awv024. Epub 2015 Mar 1. Brain. 2015. PMID: 25732182 Free PMC article.
-
Basal Forebrain and Brainstem Cholinergic Neurons Differentially Impact Amygdala Circuits and Learning-Related Behavior.Curr Biol. 2018 Aug 20;28(16):2557-2569.e4. doi: 10.1016/j.cub.2018.06.064. Epub 2018 Aug 9. Curr Biol. 2018. PMID: 30100338
-
Immunolesion by 192IgG-saporin of rat basal forebrain cholinergic system: a useful tool to produce cortical cholinergic dysfunction.Prog Brain Res. 1996;109:253-64. doi: 10.1016/s0079-6123(08)62109-3. Prog Brain Res. 1996. PMID: 9009714 Review.
-
Chemical genetic activation of the cholinergic basal forebrain hippocampal circuit rescues memory loss in Alzheimer's disease.Alzheimers Res Ther. 2022 Apr 13;14(1):53. doi: 10.1186/s13195-022-00994-w. Alzheimers Res Ther. 2022. PMID: 35418161 Free PMC article.
Cited by
-
Orexin/Hypocretin Modulates Neuroinflammatory Response to LPS in a Sex and Brain-Region Specific Manner in Young Rats.J Neurochem. 2025 Aug;169(8):e70175. doi: 10.1111/jnc.70175. J Neurochem. 2025. PMID: 40728035 Free PMC article.
-
Vulnerability of long-range inputome of basal forebrain in normal aging mice.Front Aging Neurosci. 2025 Jul 23;17:1573906. doi: 10.3389/fnagi.2025.1573906. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40771199 Free PMC article.
References
-
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI, Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6), Neuroscience 10 (4) (1983) 1185–1201. - PubMed
-
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH, Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey, J Comp Neurol 214 (2) (1983) 170–197. - PubMed
-
- Zaborszky L, Pang K, Somogyi J, Nadasdy Z, Kallo I, The basal forebrain corticopetal system revisited, Ann N Y Acad Sci 877 (1 ADVANCING FRO) (1999) 339–367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

