Caveolin-3 and Caveolae regulate ventricular repolarization
- PMID: 36842733
- PMCID: PMC10065933
- DOI: 10.1016/j.yjmcc.2023.02.005
Caveolin-3 and Caveolae regulate ventricular repolarization
Abstract
Rationale: Flask-shaped invaginations of the cardiomyocyte sarcolemma called caveolae require the structural protein caveolin-3 (Cav-3) and host a variety of ion channels, transporters, and signaling molecules. Reduced Cav-3 expression has been reported in models of heart failure, and variants in CAV3 have been associated with the inherited long-QT arrhythmia syndrome. Yet, it remains unclear whether alterations in Cav-3 levels alone are sufficient to drive aberrant repolarization and increased arrhythmia risk.
Objective: To determine the impact of cardiac-specific Cav-3 ablation on the electrophysiological properties of the adult mouse heart.
Methods and results: Cardiac-specific, inducible Cav3 homozygous knockout (Cav-3KO) mice demonstrated a marked reduction in Cav-3 expression by Western blot and loss of caveolae by electron microscopy. However, there was no change in macroscopic cardiac structure or contractile function. The QTc interval was increased in Cav-3KO mice, and there was an increased propensity for ventricular arrhythmias. Ventricular myocytes isolated from Cav-3KO mice exhibited a prolonged action potential duration (APD) that was due to reductions in outward potassium currents (Ito, Iss) and changes in inward currents including slowed inactivation of ICa,L and increased INa,L. Mathematical modeling demonstrated that the changes in the studied ionic currents were adequate to explain the prolongation of the mouse ventricular action potential. Results from human iPSC-derived cardiomyocytes showed that shRNA knockdown of Cav-3 similarly prolonged APD.
Conclusion: We demonstrate that Cav-3 and caveolae regulate cardiac repolarization and arrhythmia risk via the integrated modulation of multiple ionic currents.
Keywords: Action potential; Arrhythmia; Cardiac repolarization; Caveolae; Ion channels.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest TJK is a consultant for Fujifilm Cellular Dynamics Incorporated.
Figures



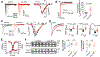


References
-
- Simons K, Toomre D, Lipid rafts and signal transduction, Nat Rev Mol Cell Biol 1(1) (2000) 31–9. - PubMed
-
- Parton RG, McMahon KA, Wu Y, Caveolae: Formation, dynamics, and function, Curr Opin Cell Biol 65 (2020) 8–16. - PubMed
-
- Razani B, Woodman SE, Lisanti MP, Caveolae: from cell biology to animal physiology, Pharmacol Rev 54(3) (2002) 431–67. - PubMed
-
- Tang Z, Scherer PE, Okamoto T, Song K, Chu C, Kohtz DS, et al. , Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle, J Biol Chem 271(4) (1996) 2255–61. - PubMed
-
- Galbiati F, Engelman JA, Volonte D, Zhang XL, Minetti C, Li M, et al. , Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities, J Biol Chem 276(24) (2001) 21425–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

