Cholestasis impairs gut microbiota development and bile salt hydrolase activity in preterm neonates
- PMID: 36843227
- PMCID: PMC9980517
- DOI: 10.1080/19490976.2023.2183690
Cholestasis impairs gut microbiota development and bile salt hydrolase activity in preterm neonates
Abstract
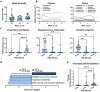
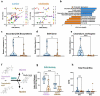
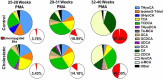
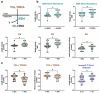
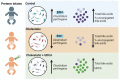
Cholestasis refers to impaired bile flow from the liver to the intestine. In neonates, cholestasis causes poor growth and may progress to liver failure and death. Normal bile flow requires an intact liver-gut-microbiome axis, whereby liver-derived primary bile acids are transformed into secondary bile acids. Microbial bile salt hydrolase (BSH) enzymes are responsible for the first step, deconjugating glycine- and taurine-conjugated primary bile acids. Cholestatic neonates often are treated with the potent choleretic bile acid ursodeoxycholic acid (UDCA), although interactions between UDCA, gut microbes, and other bile acids are poorly understood. To gain insight into how the liver-gut-microbiome axis develops in extreme prematurity and how cholestasis alters this maturation, we conducted a nested case-control study collecting 124 stool samples longitudinally from 24 preterm infants born at mean 27.2 ± 1.8 weeks gestation and 946 ± 249.6 g, half of whom developed physiologic cholestasis. Samples were analyzed by whole metagenomic sequencing, in vitro BSH enzyme activity assays optimized for low biomass fecal samples, and quantitative mass spectrometry to measure the bile acid metabolome. In extremely preterm neonates, acquisition of the secondary bile acid biosynthesis pathway and BSH genes carried by Clostridium perfringens are the most prominent features of early microbiome development. Cholestasis interrupts this developmental pattern. BSH gene abundance and enzyme activity are profoundly reduced in cholestatic neonates, resulting in decreased quantities of unconjugated bile acids. UDCA restores total fecal bile acid levels in cholestatic neonates, but this is due to a 522-fold increase in fecal UDCA. A majority of bile acids in early development are atypical positional and stereo-isomers of bile acids. We report novel associations linking isomeric bile acids and BSH activity to neonatal growth trajectories. These data highlight deconjugation of bile acids as a key microbial function that is acquired in early neonatal development and impaired by cholestasis.
Keywords: Microbiome; bile acids; bile salt hydrolase; cholestasis; growth; neonate; premature infant; ursodeoxycholic acid.
Conflict of interest statement
KDRS discloses equity in Asklepion Pharmaceuticals, LLC, Baltimore and Aliveris s.r.l. Italy, and is a consultant to Travere Therapeutics and Mirum Pharmaceuticals. No potential conflict of interest was reported by the other authors.
Figures

References
-
- Nastasio S, Maggiore G. Cholestasis in preterm infants: when is a yellow alert?. Ital J Pediatr. 2014;40(S2). A12, 1824-7288-40-S2–A12. doi:10.1186/1824-7288-40-S2-A12. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
