Change in Cerebrospinal Fluid Tau Microtubule Binding Region Detects Symptom Onset, Cognitive Decline, Tangles, and Atrophy in Dominantly Inherited Alzheimer's Disease
- PMID: 36843330
- PMCID: PMC10238659
- DOI: 10.1002/ana.26620
Change in Cerebrospinal Fluid Tau Microtubule Binding Region Detects Symptom Onset, Cognitive Decline, Tangles, and Atrophy in Dominantly Inherited Alzheimer's Disease
Abstract
Objective: Identifying cerebrospinal fluid measures of the microtubule binding region of tau (MTBR-tau) species that reflect tau aggregation could provide fluid biomarkers that track Alzheimer's disease related neurofibrillary tau pathological changes. We examined the cerebrospinal fluid (CSF) MTBR-tau species in dominantly inherited Alzheimer's disease (DIAD) mutation carriers to assess the association with Alzheimer's disease (AD) biomarkers and clinical symptoms.
Methods: Cross-sectional and longitudinal CSF from 229 DIAD mutation carriers and 130 mutation non-carriers had sequential characterization of N-terminal/mid-domain phosphorylated tau (p-tau) followed by MTBR-tau species and tau positron emission tomography (tau PET), other soluble tau and amyloid biomarkers, comprehensive clinical and cognitive assessments, and brain magnetic resonance imaging of atrophy.
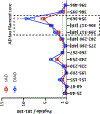
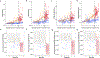
Results: CSF MTBR-tau species located within the putative "border" region and one species corresponding to the "core" region of aggregates in neurofibrillary tangles (NFTs) increased during the presymptomatic stage and decreased during the symptomatic stage. The "border" MTBR-tau species were associated with amyloid pathology and CSF p-tau; whereas the "core" MTBR-tau species were associated stronger with tau PET and CSF measures of neurodegeneration. The ratio of the border to the core species provided a continuous measure of increasing amounts that tracked clinical progression and NFTs.
Interpretation: Changes in CSF soluble MTBR-tau species preceded the onset of dementia, tau tangle increase, and atrophy in DIAD. The ratio of 4R-specific MTBR-tau (border) to the NFT (core) MTBR-tau species corresponds to the pathology of NFTs in DIAD and change with disease progression. The dynamics between different MTBR-tau species in the CSF may serve as a marker of tau-related disease progression and target engagement of anti-tau therapeutics. ANN NEUROL 2023;93:1158-1172.
© 2023 American Neurological Association.
Conflict of interest statement
Potential Conflict of Interests:
There are several inventions that have been filed by Washington University for patents, including “Methods of diagnosing AD with phosphorylation changes” and “Methods to detect MTBR-tau isoforms and use”. These intellectual properties owned by Washington University can be or are licensed and some licensing income may be distributed to Drs. Barthelemy, Bateman, Horie, McDade and Sato and other inventors. These intellectual properties being licensed by Washington University from C2N and currently being utilized in our research have been reviewed by the Washington University COI and ICOI committees.
All co-inventors, including some lab members, the University, and Drs. Barthelemy, Bateman, Horie, McDade and Sato could receive part of the profits from any sales of these tests by C2N, which is in the process of licensing or has licensed some IP from the University. These activities have been reviewed by Washington University’s (WU) Conflicts of Interest Review Committee in accordance with WU’s Research Conflicts of Interest Policy and WU’s Institutional Conflict of Interest Review Committee in accordance with WU’s Institutional Conflict of Interest Policy.
K.H. is an Eisai-sponsored voluntary research associate professor at Washington University and has received salary from Eisai.
RJB has received research funding from Avid Radiopharmaceuticals, Janssen, Roche/Genentech, Eli Lilly, Eisai, Biogen, AbbVie, Bristol Myers Squibb, and Novartis. Washington University and RJB have equity ownership interest in C2N Diagnostics and receive income based on technology (stable isotope labeling kinetics, blood plasma assay, and methods of diagnosing AD with phosphorylation changes) licensed by Washington University to C2N Diagnostics. RJB receives income from C2N Diagnostics for serving on the scientific advisory board. RJB serves on the Roche Gantenerumab Steering Committee as an unpaid member.
JL reports speaker fees from Bayer Vital, Biogen and Roche, consulting fees from Axon Neuroscience and Biogen, author fees from Thieme medical publishers and W. Kohlhammer GmbH medical publishers, non-financial support from Abbvie and compensation for duty as part-time CMO from MODAG and being beneficiary of the phantom share program of MODAG GmbH, outside the submitted work.
Figures


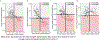
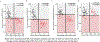
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

