Preparation and in vivo evaluation of an intravenous emulsion loaded with an aprepitant-phospholipid complex
- PMID: 36843571
- PMCID: PMC9979997
- DOI: 10.1080/10717544.2023.2183834
Preparation and in vivo evaluation of an intravenous emulsion loaded with an aprepitant-phospholipid complex
Abstract
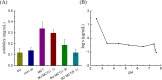
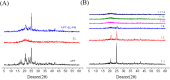
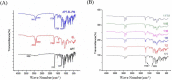
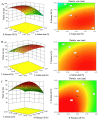
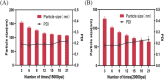
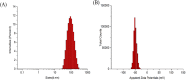
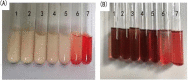
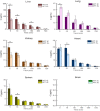
In present, there was no detailed report on the formulation optimization and quality evaluation of aprepitant (APT) injectable lipid emulsion (APT-IE). The aim of the present investigation was to prepare and evaluate its properties of APT-IE loaded with an APT phospholipid complex (APT-PC) in vitro and in vivo. APT-PC was obtained by solvent evaporation with APT and phospholipids, then analyzed by X-ray diffraction, Fourier transform infrared spectroscopy and differential scanning calorimetry. Lipid emulsions are a new formulation that can reduce side effects and improve drug loading.APT-IE prepared by High-pressure homogenization and optimized by response surface methodology (RSM). The proportion of sodium oleate, poloxamer 188 and soybean oil were selected as variables for the optimization. The optimal formulation of ATP-IE had the following characteristics: particle size, 82.83 ± 1.89 nm; polydispersity index, 0.243 ± 0.008; zeta potential, -59.0 ± 2.54 mV; encapsulation efficiency, 98.84%±1.43%; drug loading, 7.08 ± 0.16 mg/mL; and osmotic pressure, 301 ± 2.15 mOsmol/kg. Transmission electron microscopy images indicated that the particle diameter of APT-IE was approximately 100 nm, with a morphology of spheroidal or spherical. APT-IE exhibited sufficient stability after storage at 4 ± 2 °C for more than 6 months. The results of the pharmacokinetic study demonstrated that APT-IE had the advantages of better safety, higher bioavailability, and obvious liver targeting than APT solution (APT-SL). The area under the curve (AUC) of APT-IE was 3-fold enhanced compared with APT-SL. The targeted enhancement multiple of APT-IE to liver tissue was greater than that of APT-SL. These results suggested that APT-IE has broad clinical application and industrial production potential.
Keywords: Aprepitant; lipid injectable emulsion; pharmacokinetics; response surface methodology; tissue distribution.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures




References
-
- Augustyn B, Stepien P, Poojari C, et al. (2019). Cholesteryl hemisuccinate is not a good replacement for cholesterol in lipid nanodiscs. J Phys Chem B 123:9839–45. - PubMed
-
- Carpentier YA, Dupont IE. (2000). Advances in intravenous lipid emulsions. World J Surg 24:1493–7. - PubMed
-
- Chu T, Zhang Q, Li H, et al. (2012). Development of intravenous lipid emulsion of tanshinone IIA and evaluation of its anti-hepatoma activity in vitro. Int J Pharm 42:76–88. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
