Interactions between β-arrestin proteins and the cytoskeletal system, and their relevance to neurodegenerative disorders
- PMID: 36843600
- PMCID: PMC9947276
- DOI: 10.3389/fendo.2023.957981
Interactions between β-arrestin proteins and the cytoskeletal system, and their relevance to neurodegenerative disorders
Abstract
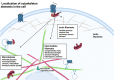
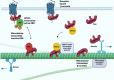
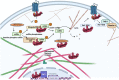
β-arrestins, which have multiple cellular functions, were initially described as proteins that desensitize rhodopsin and other G protein-coupled receptors. The cytoskeletal system plays a role in various cellular processes, including intracellular transport, cell division, organization of organelles, and cell cycle. The interactome of β-arrestins includes the major proteins of the three main cytoskeletal systems: tubulins for microtubules, actins for the actin filaments, and vimentin for intermediate filaments. β-arrestins bind to microtubules and regulate their activity by recruiting signaling proteins and interacting with assembly proteins that regulate the actin cytoskeleton and the intermediate filaments. Altered regulation of the cytoskeletal system plays an essential role in the development of Alzheimer's, Parkinson's and other neurodegenerative diseases. Thus, β-arrestins, which interact with the cytoskeleton, were implicated in the pathogenesis progression of these diseases and are potential targets for the treatment of neurodegenerative disorders in the future.
Keywords: Alzheimer’s disease; actin; arrestin; cytoskeleton; microtubule; neurodegeneration.
Copyright © 2023 Szénási, Turu and Hunyady.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

