Thyroid hormone action controls multiple components of cell junctions at the ventricular zone in the newborn rat brain
- PMID: 36843608
- PMCID: PMC9950412
- DOI: 10.3389/fendo.2023.1090081
Thyroid hormone action controls multiple components of cell junctions at the ventricular zone in the newborn rat brain
Abstract
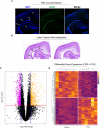
Thyroid hormone (TH) action controls brain development in a spatiotemporal manner. Previously, we demonstrated that perinatal hypothyroidism led to formation of a periventricular heterotopia in developing rats. This heterotopia occurs in the posterior telencephalon, and its formation was preceded by loss of radial glia cell polarity. As radial glia mediate cell migration and originate in a progenitor cell niche called the ventricular zone (VZ), we hypothesized that TH action may control cell signaling in this region. Here we addressed this hypothesis by employing laser capture microdissection and RNA-Seq to evaluate the VZ during a known period of TH sensitivity. Pregnant rats were exposed to a low dose of propylthiouracil (PTU, 0.0003%) through the drinking water during pregnancy and lactation. Dam and pup THs were quantified postnatally and RNA-Seq of the VZ performed in neonates. The PTU exposure resulted in a modest increase in maternal thyroid stimulating hormone and reduced thyroxine (T4). Exposed neonates exhibited hypothyroidism and T4 and triiodothyronine (T3) were also reduced in the telencephalon. RNA-Seq identified 358 differentially expressed genes in microdissected VZ cells of hypothyroid neonates as compared to controls (q-values ≤0.05). Pathway analyses showed processes like maintenance of the extracellular matrix and cytoskeleton, cell adhesion, and cell migration were significantly affected by hypothyroidism. Immunofluorescence also demonstrated that collagen IV, F-actin, radial glia, and adhesion proteins were reduced in the VZ. Immunohistochemistry of integrin αvβ3 and isoforms of both thyroid receptors (TRα/TRβ) showed highly overlapping expression patterns, including enrichment in the VZ. Taken together, our results show that TH action targets multiple components of cell junctions in the VZ, and this may be mediated by both genomic and nongenomic mechanisms. Surprisingly, this work also suggests that the blood-brain and blood-cerebrospinal fluid barriers may also be affected in hypothyroid newborns.
Keywords: brain development; cell adhesion; cell junctions; cell migration; hypothyroidism; radial glia; thyroid hormone action; ventricular zone.
Copyright © 2023 O’Shaughnessy, McMichael, Sasser, Bell, Riutta, Ford, Stoker, Grindstaff, Pandiri and Gilbert.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Bernal J. Thyroid hormones in brain development and function. In: De Groot LJ, Beck-Peccoz GCP, Dungan K, Grossman A, Hershman JM, C. Koch R, McLachlan MN, Rebar R, Singer F, Vinik A, Weickert MO, editors. Endotext. South Dartmouth (MA: MDText.com, Inc; (2015).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

