Chemistry and Pharmacological diversity of Benzothiazepine - Excellent pathway to drug discovery
- PMID: 36843650
- PMCID: PMC9957176
- DOI: 10.1016/j.molstruc.2023.135071
Chemistry and Pharmacological diversity of Benzothiazepine - Excellent pathway to drug discovery
Abstract
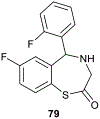
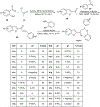
In this era of sporadic advancement in science and technology, a substantial amount of intervention is being set in motion to reduce health-related diseases. Discoveries from researchers have pinpointed the usefulness of heterocyclic compounds, amongst which benzothiazepine (BTZ) derivatives have been synthesized for their various pharmacological activities. This also contributes to their undeniable application in therapeutic medicine for the development of efficacious drugs. BTZs are compounds with a benzene ring fused with a thiazepine ring. This work contains several methods that have been used to synthesize 1,3-, 1,4-, 1,5-, and 4-1-benzothiazepine derivatives. In addition, up-to-date information about the crucial pharmacological activities of BTZ derivatives has been reviewed in this present study to appreciate their druggable potential in therapeutic medicine for drug development. Drug design and development have further been simplified with the implementation of computer aided approaches to predict biological interactions which can help in the design of several derivatives. Hence, the structural activity relationship (SAR), ADMET and the molecular docking studies of BTZ derivatives were discussed to further establish their interactions and safety in biological systems. This present work aims to expound on the reported chemistry and pharmacological propensity of BTZ moiety in relation to other relevant moieties to validate their potential as excellent pharmacophores in drug design and development.
Keywords: ADMET; Drug design; Heterocyclic compounds; Medicinal properties; Molecular docking; Synthesis.
Conflict of interest statement
Conflict of interest: The authors declare no potential conflict of interest concerning the research, authorship, and/or publication of this article.
Figures
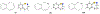
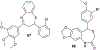




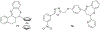













Similar articles
-
Advancing drug discovery: Thiadiazole derivatives as multifaceted agents in medicinal chemistry and pharmacology.Bioorg Med Chem. 2024 Oct 1;112:117876. doi: 10.1016/j.bmc.2024.117876. Epub 2024 Aug 16. Bioorg Med Chem. 2024. PMID: 39163743 Review.
-
Structure activity relationship (SAR) study of benzimidazole scaffold for different biological activities: A mini-review.Eur J Med Chem. 2015 Jun 5;97:419-43. doi: 10.1016/j.ejmech.2014.11.053. Epub 2014 Nov 26. Eur J Med Chem. 2015. PMID: 25479684 Review.
-
Synthesis of Azoloquinazolines and Substituted Benzothiazepine as Antimicrobial Agents.Mini Rev Med Chem. 2020;20(5):418-429. doi: 10.2174/1389557519666190603091101. Mini Rev Med Chem. 2020. PMID: 31161988
-
Japan-China Joint Medical Workshop on Drug Discoveries and Therapeutics 2008: The need of Asian pharmaceutical researchers' cooperation.Drug Discov Ther. 2008 Oct;2(5):262-3. Drug Discov Ther. 2008. PMID: 22504718
-
Morpholine as ubiquitous pharmacophore in medicinal chemistry: Deep insight into the structure-activity relationship (SAR).Bioorg Chem. 2020 Mar;96:103578. doi: 10.1016/j.bioorg.2020.103578. Epub 2020 Jan 11. Bioorg Chem. 2020. PMID: 31978684 Review.
Cited by
-
Investigation of photophysical properties and potential biological applications of substituted tris(polypyridyl)ruthenium(II) complexes.Front Chem. 2025 Feb 3;13:1491598. doi: 10.3389/fchem.2025.1491598. eCollection 2025. Front Chem. 2025. PMID: 39963354 Free PMC article.
References
-
- Zlotin SG, Churakov AM, Dalinger IL, et al. Recent advances in synthesis of organic nitrogen–oxygen systems for medicine and materials science. Mendeleev Commun. 2017;27(6):535–546. doi:10.1016/J.MENCOM.2017.11.001 - DOI
-
- Yet L. Introduction. In: Privileged Structures in Drug Discovery: Medicinal Chemistry and Synthesis. First. Jonh Wiley & Sons, Inc.; 2018:1–14.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

