Transgenerational epigenetic inheritance of axonal regeneration after spinal cord injury
- PMID: 36843857
- PMCID: PMC9949995
- DOI: 10.1093/eep/dvad002
Transgenerational epigenetic inheritance of axonal regeneration after spinal cord injury
Abstract
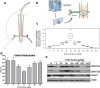
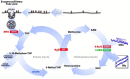
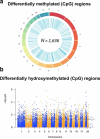
Human epidemiological studies reveal that dietary and environmental alterations influence the health of the offspring and that the effect is not limited to the F1 or F2 generations. Non-Mendelian transgenerational inheritance of traits in response to environmental stimuli has been confirmed in non-mammalian organisms including plants and worms and are shown to be epigenetically mediated. However, transgenerational inheritance beyond the F2 generation remains controversial in mammals. Our lab previously discovered that the treatment of rodents (rats and mice) with folic acid significantly enhances the regeneration of injured axons following spinal cord injury in vivo and in vitro, and the effect is mediated by DNA methylation. The potential heritability of DNA methylation prompted us to investigate the following question: Is the enhanced axonal regeneration phenotype inherited transgenerationally without exposure to folic acid supplementation in the intervening generations? In the present review, we condense our findings showing that a beneficial trait (i.e., enhanced axonal regeneration after spinal cord injury) and accompanying molecular alterations (i.e., DNA methylation), triggered by an environmental exposure (i.e., folic acid supplementation) to F0 animals only, are inherited transgenerationally and beyond the F3 generation.
Keywords: CNS regeneration; DNA methylation; epigenetics; folate; folic acid; one carbon metabolism.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
None declared.
Figures

References
-
- Iskandar BJ, Nelson A, Resnick D et al. Folic acid supplementation enhances repair of the adult central nervous system. Ann Neurol 2004;56:221–7. - PubMed
-
- Selzer RR, Rosenblatt DS, Laxova R et al. Adverse effect of nitrous oxide in a child with 5,10-methylenetetrahydrofolate reductase deficiency. N Engl J Med 2003;349:45–50. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

