Bu-Fei-Huo-Xue capsule alleviates bleomycin-induced pulmonary fibrosis in mice through modulating gut microbiota
- PMID: 36843927
- PMCID: PMC9944029
- DOI: 10.3389/fphar.2023.1084617
Bu-Fei-Huo-Xue capsule alleviates bleomycin-induced pulmonary fibrosis in mice through modulating gut microbiota
Abstract
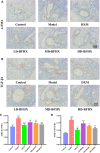
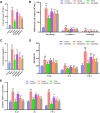
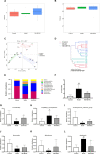
Introduction: Bu-Fei-Huo-Xue capsule (BFHX) has been used to treat pulmonary fibrosis (PF) in clinic. However, the mechanism of Bu-Fei-Huo-Xue capsule on pulmonary fibrosis remains unclear. Recent studies have shown that the changes in gut microbiota were closely related to the progression of pulmonary fibrosis. Modulating gut microbiota provides new thoughts in the treatment of pulmonary fibrosis. Methods: In this study,a mouse model of pulmonary fibrosis was induced using bleomycin (BLM) and treated with Bu-Fei-Huo-Xue capsule. We firstly evaluated the therapeutic effects of Bu-Fei-Huo-Xue capsule on pulmonary fibrosis model mice. Besides,the anti-inflammatory and anti- oxidative effects of Bu-Fei-Huo-Xue capsule were evaluated. Furthermore, 16S rRNA sequencing was used to observe the changes in gut microbiota in pulmonary fibrosis model mice after Bu-Fei-Huo-Xue capsule treatment. Results: Our results showed that Bu-Fei-Huo-Xue capsule significantly reduced the collagen deposition in pulmonary fibrosis model mice. Bu-Fei-Huo-Xue capsule treatment also reduced the levels and mRNA expression of pro-inflammatory cytokines and inhibited the oxidative stress in lung. 16S rRNA sequencing showed that Bu-Fei-Huo-Xue capsule affected the diversity of gut microbiota and the relative abundances of gut microbiota such as Lactobacillus, Lachnospiraceae_NK4A136_group, and Romboutsia. Conclusion: Our study demonstrated the therapeutic effects of Bu-Fei-Huo-Xue capsule on pulmonary fibrosis. The mechanisms of Bu-Fei-Huo-Xue capsule on pulmonary fibrosis may be associated with regulating gut microbiota.
Keywords: Bu-Fei-Huo-Xue capsule; gut microbiota; inflammation; oxidative stress; pulmonary fibrosis.
Copyright © 2023 Hu, Wang, Han, Li, Wang, Song, Zhao, Li, Lu, Tao and Cui.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Yi-Fei-Tong-Bi Decoction Alleviates Bleomycin Induced Pulmonary Fibrosis in Mice.Drug Des Devel Ther. 2025 May 15;19:3983-3995. doi: 10.2147/DDDT.S515368. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40391179 Free PMC article.
-
Qi-Long-Tian capsule alleviates pulmonary fibrosis development by modulating inflammatory response and gut microbiota.Funct Integr Genomics. 2023 Feb 22;23(1):64. doi: 10.1007/s10142-023-00988-3. Funct Integr Genomics. 2023. PMID: 36810971
-
Protective effects of Qing-Re-Huo-Xue formula on bleomycin-induced pulmonary fibrosis through the p53/IGFBP3 pathway.Chin Med. 2023 Mar 30;18(1):33. doi: 10.1186/s13020-023-00730-y. Chin Med. 2023. PMID: 36997948 Free PMC article.
-
Polydatin alleviates bleomycin-induced pulmonary fibrosis and alters the gut microbiota in a mouse model.J Cell Mol Med. 2023 Dec;27(23):3717-3728. doi: 10.1111/jcmm.17937. Epub 2023 Sep 4. J Cell Mol Med. 2023. PMID: 37665061 Free PMC article.
-
A Chinese classical prescription Maimendong decoction in treatment of pulmonary fibrosis: an overview.Front Pharmacol. 2024 May 9;15:1329743. doi: 10.3389/fphar.2024.1329743. eCollection 2024. Front Pharmacol. 2024. PMID: 38783956 Free PMC article. Review.
Cited by
-
Triangle correlations of lung microbiome, host physiology and gut microbiome in a rat model of idiopathic pulmonary fibrosis.Sci Rep. 2024 Nov 20;14(1):28743. doi: 10.1038/s41598-024-80023-y. Sci Rep. 2024. PMID: 39567656 Free PMC article.
-
Quercetin influences intestinal dysbacteriosis and delays alveolar epithelial cell senescence by regulating PTEN/PI3K/AKT signaling in pulmonary fibrosis.Naunyn Schmiedebergs Arch Pharmacol. 2024 Jul;397(7):4809-4822. doi: 10.1007/s00210-023-02913-8. Epub 2023 Dec 28. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38153514 Free PMC article.
-
A Subgroup Reanalysis of the Efficacy of Bufei Huoxue Capsules in Patients With "Long-Covid-19".Pulm Circ. 2025 Apr 27;15(2):e70084. doi: 10.1002/pul2.70084. eCollection 2025 Apr. Pulm Circ. 2025. PMID: 40291435 Free PMC article.
-
The mechanism of gut-lung axis in pulmonary fibrosis.Front Cell Infect Microbiol. 2024 Feb 1;14:1258246. doi: 10.3389/fcimb.2024.1258246. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38362497 Free PMC article. Review.
-
Assessing the impact of triiodothyronine treatment on the lung microbiome of mice with pulmonary fibrosis.BMC Pulm Med. 2024 Aug 23;24(1):405. doi: 10.1186/s12890-024-03214-3. BMC Pulm Med. 2024. PMID: 39180004 Free PMC article.
References
-
- Bhattacharya S. S., Yadav B., Rosen L., Nagpal R., Yadav H., Yadav J. S. (2022). Crosstalk between gut microbiota and lung inflammation in murine toxicity models of respiratory exposure or co-exposure to carbon nanotube particles and cigarette smoke extract. Toxicol. Appl. Pharmacol. 447, 116066. 10.1016/j.taap.2022.116066 - DOI - PubMed
-
- Cruz B. C., Dos S., Conceição L. L., Mendes T. A., Ferreira C. L., Gonçalves R. V., et al. (2020). Use of the synbiotic VSL#3 and yacon-based concentrate attenuates intestinal damage and reduces the abundance of Candidatus Saccharimonas in a colitis-associated carcinogenesis model. Food Res. Int. 137, 109721. 10.1016/j.foodres.2020.109721 - DOI - PubMed
LinkOut - more resources
Full Text Sources

