Pharmacovigilance on cannabidiol as an antiepileptic agent
- PMID: 36843933
- PMCID: PMC9950105
- DOI: 10.3389/fphar.2023.1091978
Pharmacovigilance on cannabidiol as an antiepileptic agent
Abstract
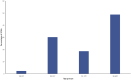
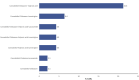
Introduction: Cannabidiol (CBD) is an active chemical contained in the plant Cannabis sativa. It is a resorcinol-based compound that crosses the blood-brain barrier without causing euphoric effects. CBD has a plethora of pharmacological effects of therapeutic interest. CBD has been authorized in the European Union as an anticonvulsant against serious infantile epileptic syndromes, but its safety profile is still not sufficiently described. Methods: With the goal of expanding information on the safety of CBD use as an antiepileptic agent beyond the most common side effects known through clinical studies, an analysis of serious case reports on suspected adverse reactions (SARs) to CBD licensed as an anti-epileptic drug found in the EudraVigilance database is reported in this article. EudraVigilance is a system purchased by the European Medicines Agency (EMA) for monitoring the safety of medicinal products marketed in Europe. Results: The most frequent serious SARs to CBD in EudraVigilance were epilepsy aggravation, hepatic disorders, lack of efficacy, and somnolence. Discussion: Based on our analysis, the following precautions should be adopted for appropriate monitoring of potential adverse effects, more attention towards possible CBD medical use as an antiepileptic: awareness of interactions with other drugs, epilepsy aggravation, and drug effectiveness.
Keywords: adverse reactions; cannabidiol; cannabis; epilepsy; pharmacovigilance.
Copyright © 2023 Ammendolia, Mannucci, Cardia, Calapai, Gangemi, Esposito and Calapai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Pharmacovigilance of unlicensed cannabidiol in European countries.Phytother Res. 2024 Jan;38(1):74-81. doi: 10.1002/ptr.8028. Epub 2023 Oct 6. Phytother Res. 2024. PMID: 37800192
-
Highly Purified Cannabidiol for Epilepsy Treatment: A Systematic Review of Epileptic Conditions Beyond Dravet Syndrome and Lennox-Gastaut Syndrome.CNS Drugs. 2021 Mar;35(3):265-281. doi: 10.1007/s40263-021-00807-y. Epub 2021 Mar 22. CNS Drugs. 2021. PMID: 33754312 Free PMC article.
-
Source of cannabinoids: what is available, what is used, and where does it come from?Epileptic Disord. 2020 Jan 1;22(S1):1-9. doi: 10.1684/epd.2019.1121. Epileptic Disord. 2020. PMID: 31941643
-
Cannabis-based medicines--GW pharmaceuticals: high CBD, high THC, medicinal cannabis--GW pharmaceuticals, THC:CBD.Drugs R D. 2003;4(5):306-9. doi: 10.2165/00126839-200304050-00005. Drugs R D. 2003. PMID: 12952500 Review.
-
Don't Fear the Reefer-Evidence Mounts for Plant-Based Cannabidiol as Treatment for Epilepsy.Epilepsy Curr. 2019 Mar-Apr;19(2):93-95. doi: 10.1177/1535759719835671. Epilepsy Curr. 2019. PMID: 30955420 Free PMC article.
Cited by
-
Therapeutic Approaches to Tuberous Sclerosis Complex: From Available Therapies to Promising Drug Targets.Biomolecules. 2024 Sep 21;14(9):1190. doi: 10.3390/biom14091190. Biomolecules. 2024. PMID: 39334956 Free PMC article. Review.
-
Pharmacological Evaluation of Signals of Disproportionality Reporting Related to Adverse Reactions to Antiepileptic Cannabidiol in VigiBase.Pharmaceuticals (Basel). 2023 Oct 5;16(10):1420. doi: 10.3390/ph16101420. Pharmaceuticals (Basel). 2023. PMID: 37895891 Free PMC article.
-
Overview of global monitoring systems for the side effects and adverse events associated with medicinal cannabis use: a scoping review using a systematic approach.BMJ Open. 2024 Jul 18;14(7):e085166. doi: 10.1136/bmjopen-2024-085166. BMJ Open. 2024. PMID: 39025811 Free PMC article.
-
Suspected oncologic adverse reactions associated with interleukin-23 inhibitors in EudraVigilance: Comparative study and gender distribution.Pharmacol Res Perspect. 2023 Oct;11(5):e01130. doi: 10.1002/prp2.1130. Pharmacol Res Perspect. 2023. PMID: 37615258 Free PMC article.
-
The Polypharmacological Effects of Cannabidiol.Molecules. 2023 Apr 6;28(7):3271. doi: 10.3390/molecules28073271. Molecules. 2023. PMID: 37050032 Free PMC article. Review.
References
-
- Atalay S., Gęgotek A., Wroński A., Domigues P., Skrzydlewska E. (2021). Corrigendum to "Therapeutic application of cannabidiol on UVA and UVB irradiated rat skin. A proteomic study" [J. Pharm. Biomed. Anal. 192 (2021) 1-9/113656]. J. Pharm. Biomed. Anal. 200, 114038. 10.1016/j.jpba.2021.114038 - DOI - PubMed
-
- Boulebd H., Pereira D. M., Khodja I. A., Hoa N. T., Mechler A., Vo Q. V. (2022). Assessment of the free radical scavenging potential of cannabidiol under physiological conditions: Theoretical and experimental investigations. J. Mol. Liq. 346, 118277. 10.1016/j.molliq.2021.118277 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

