Continuous Flow Epoxidation of Alkenes Using a Homogeneous Manganese Catalyst with Peracetic Acid
- PMID: 36844035
- PMCID: PMC9942194
- DOI: 10.1021/acs.oprd.2c00222
Continuous Flow Epoxidation of Alkenes Using a Homogeneous Manganese Catalyst with Peracetic Acid
Abstract
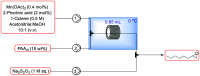
Epoxidation of alkenes is a valuable transformation in the synthesis of fine chemicals. Described herein are the design and development of a continuous flow process for carrying out the epoxidation of alkenes with a homogeneous manganese catalyst at metal loadings as low as 0.05 mol%. In this process, peracetic acid is generated in situ and telescoped directly into the epoxidation reaction, thus reducing the risks associated with its handling and storage, which often limit its use at scale. This flow process lessens the safety hazards associated with both the exothermicity of this epoxidation reaction and the use of the highly reactive peracetic acid. Controlling the speciation of manganese/2-picolinic acid mixtures by varying the ligand:manganese ratio was key to the success of the reaction. This continuous flow process offers an inexpensive, sustainable, and scalable route to epoxides.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Moschona F.; Savvopoulou I.; Tsitopoulou M.; Tataraki D.; Rassias G. Epoxide Syntheses and Ring-Opening Reactions in Drug Development. Catalysts 2020, 10 (10), 1117. 10.3390/catal10101117. - DOI
-
- Thirumalaikumar M. Ring Opening Reactions of Epoxides. A Review. Org. Prep. Proced. Int. 2022, 54 (1), 1–39. 10.1080/00304948.2021.1979357. - DOI
Publication types
LinkOut - more resources
Full Text Sources